Translate this page into:
Clinical, Microbiological Profile, and Treatment Outcomes of Carbapenem-Resistant Urinary Tract Infections in a Tertiary Care Hospital
Corresponding author: Rahul Sai Gangula, Department of Nephrology, M.S. Ramaiah Medical College, India. Email: rahulsai1990@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Eshwarappa M, Gangula RS, Rajashekar R, Prabhu PP, Hamsa V, Yousuff M, et al. Clinical, Microbiological Profile, and Treatment Outcomes of Carbapenem-Resistant Urinary Tract Infections in a Tertiary Care Hospital. Indian J Nephrol. 2025;35:53-8. doi: 10.25259/ijn_530_23
Abstract
Background
Carbapenem-resistant urinary tract infections (CR-UTIs) are a major global health threat. Many factors contribute to the increasing incidence of CR-UTI. Owing to the limited availability of treatment options, CR-UTIs are highly challenging to treat.
Materials and Methods
This was a single-center, hospital-based, observational, retrospective cohort study. We investigated the treatment results, microbiological profiles, and clinical manifestations of CR-UTI at our institution between January 2017 and December 2021. All patients exhibiting clinical signs and symptoms of urinary tract infection (UTI) and a urine culture that showed growth of a single organism greater than 105 colony-forming units/ml were included. All patients were considered for a 1-year follow-up.
Results
From January 2017 to December 2022, 3016 (31%) CR-UTI episodes were noted. Approximately, 75% of CR-UTI episodes were caused by the most prevalent urinary pathogens, Escherichia coli and Klebsiella pneumoniae. Within 28 days, 308 patients (12.59%) died. Enterobacteriaceae treated for a minimum of 7–10 days showed a greater response to Aminoglycosides, Fosfomycin, Ceftizoxime, Colistin with Carbapenem, Tigecycline with Carbapenem, and Ceftazidime/avibactam. Within a year, 994 CR-UTI episodes were identified in patients who were available for follow-up; and 38% of these episodes were the result of relapse. Three-quarters of the remaining incidents were recurrent, accounting for a higher mortality rate (14.2%) within a year.
Conclusion
Despite effective antibiotic treatment, CR-UTIs are associated with early relapse and recurrence. Newer effective treatment and preventive strategies are required to address this pandemic.
Keywords
Carbapenem-sensitive
Carbapenem-resistance
Recurrent
Non-recurrent
Relapse
Re-infection
Introduction
Urinary tract infection (UTI) is defined as a patient with clinical symptoms and signs of UTI, with a clean catch mid-stream urine culture sample revealing single microorganism growth of >105 bacterial colony-forming units/ml.1 Gram-negative bacteria are the predominant organisms causing UTIs, of which Escherichia coli accounts for approximately 90% of the cases.2-4 When treating challenging infections caused by Enterobacteriaceae that produce extended-spectrum β-lactamases, carbapenems, and broad-spectrum antibiotics are routinely utilized.5 Carbapenem resistance in Enterobacteriaceae was uncommon before 2000.6,7 However, Carbapenem hydrolyzing enzymes that confer drug resistance have been identified in Klebsiella pneumoniae and other Enterobacteriaceae in recent years.5-7 From 1998 to 2014, the global prevalence of Carbapenem resistance ranged from 2% to 53%.6,7 The transfer of resistance genes through plasmids contributes to the emergence and spread of Carbapenem-resistant bacteria, often called carbapenem-resistant enterobacteriaceae (CRE).8
Risk factors for CRE infection include recent healthcare exposure or hospitalization (particularly intensive care unit admission), surgery, dialysis, presence of an indwelling catheter, mechanical ventilation, poor functional status, long-term care admission, and exposure to broad-spectrum antimicrobials.9 Antibiotics associated with CRE acquisition (either colonization or infection) include carbapenems, cephalosporins, fluoroquinolones, and vancomycin.9 In patients with Carbapenem-resistant illnesses, removal of the source of the infection is associated with better survival.6,9
Carbapenem-resistant urinary tract infections (CR-UTIs) are becoming increasingly prevalent, are difficult to treat, and can result in serious consequences.10 CRE isolation is linked to 29%–52% of all-cause hospital mortality.6.9,10 It is difficult to assess the outcomes associated with CRE infection as most of the studies were retrospective and it is difficult to distinguish between infection and colonization.9,10 Preventing the spread of Carbapenem-resistant bacteria requires a multifaceted approach that includes infection control measures, surveillance systems, antibiotic stewardship programs, and the development of new antibiotics and treatment strategies.10
Owing to these shortcomings, the current investigation aids in the identification of risk factors and effective treatment approaches that may be considered for the management of CR-UTIs. The objectives of this study were to evaluate the prevalence and to study the microbiological and clinical characteristics of CR-UTI, determine the treatment outcomes, and mortality associated with CR-UTI and evaluate the prevalence of relapse and reinfection among patients with CR-UTIs.
Materials and Methods
This single-center, hospital-based, observational, retrospective cohort study was conducted at our tertiary care hospital after obtaining approval from the Institutional Review Board at M S Ramaiah Medical College, number MSRMC/EC/SP-02/02-2-23, DRP/IFP975/2022, dated 22-02-2023. The study included all patients who visited the outpatient department or were admitted to the hospital with symptoms of UTI and had UTI confirmed by positive urine culture reports during the enrollment period i.e., from January 1, 2017 to December 31, 2021. All patients were followed up for a minimum of 1 year (i.e., until December 2022). Since, it was a retrospective study, patient consent was not required.
UTI is defined as the presence of clinical symptoms, signs of UTI, and a urine culture sample revealing single microorganism growth of >105 bacterial colony-forming units/ml.
Recurrent UTIs are defined as 3 or more UTIs in any consecutive 12 months (or) 2 or more UTIs in any consecutive 6 months, irrespective of the causative organism.
Reinfection is defined as a new infection, either by the same bacteria or by different bacteria, which occur after 2 weeks of treatment of the initial infection and after an intervening sterile urine culture. Relapse is defined as a repeat infection occurring within, or at 2 weeks after treatment which is caused by the same bacteria as the initial infection.
Isolation and identification of urinary pathogens
Approximately, 50 ml of urine specimens were collected in a sterile wide-mouth leak-proof container. Agar plates were streaked with 10 μl of the uncentrifuged specimen using a modified Mayo procedure, without burning the loop for isolation. The entire process was performed for 24 h at 35–37°C using the calibrated loop method with a loop diameter of 4 mm. If a single organism grew at a concentration of more than 105 colony-forming units/ml, the specimen was deemed positive for urinary tract infection. Culture isolates of Gram-positive and Gram-negative organisms were used, and different biochemical reactions were used to identify the organisms at the genus and species levels where necessary.
Antibiotic sensitivity testing
Following the criteria of the Clinical and Laboratory Standards Institute (CLSI), antibiotic sensitivity testing was conducted in the event of any possible growth using either the modified Kirby-Bauer disc diffusion method or the automated VITEK MS Advanced Expert System. Automated advanced expert system instruments were used to assess antibiotic susceptibility testing with a defined inoculum for the test strain and diluted in a specific broth with a drop of antimicrobial susceptibility testing indicator added to automated systems. The turbidity of the final inoculum was adjusted to 0.5 McFarland Standard. The inoculum was poured into a panel, fastened, and the inoculated panel was inserted into a VITEK MS automated system instrument. The instrument then reads the panel automatically, and the data collected were evaluated using preliminary algorithms and compared to controlled results.
Antibiotic resistance was defined according to CLSI guidelines4 for antimicrobial susceptibility testing.
Carbapenem resistance was defined as non-susceptibility to at least one Carbapenem. Non-susceptibility was defined based on CDC-defined breakpoints established by the CLSI [Table S1].
For bacteria that are intrinsically not susceptible to Imipenem (e.g., Proteus spp., Morganella spp., Providencia spp.), resistance to at least one Carbapenem other than Imipenem was required.
For bacteria that are intrinsically not susceptible to Ertapenem (e.g., Acinetobacter spp, Pseudomonas spp.), resistance to at least one Carbapenem other than Ertapenem was required.
All patients with CR-UTI were treated according to the standard of care by the treating physician after carefully evaluating the risks and benefits of various available treatment approaches.
Treatment failure was defined as patients having either clinical treatment failure, microbiological treatment failure, or both. Treatment responsive was defined as patients with neither clinical nor microbiological treatment failure.Clinical failure was defined as 28-day mortality, 28-day microbiological recurrence, or clinical worsening/failure to improve with antibiotic treatment. Microbiological failure was defined as the growth of a causative organism from repeat urine culture at greater than or equal to 5 days from index urine culture while the patient was receiving effective antibiotic therapy.
All patients who were available for follow-up were monitored for a minimum of 1 year. Patients with evidence of reinfection or relapse were treated accordingly.
The frequencies (percentages) in each category were computed to generate descriptive statistics for categorical variables. The mean and standard deviation were used to summarize the quantitative data, which had a normal distribution. The median and interquartile range were used to summarize quantitative variables with skewed distributions. The Kaplan–Meier survival analyses were used to compute and compare patient survival outcomes at the end of 28 days according to the bacteria causing CR-UTI (log-rank test, a p-value of 0.05 was considered significant). Kaplan–Meier survival analysis was computed for the patient survival outcomes at the end of 28 days during the follow-up and was stratified according to the number and type of UTI during the follow-up. Statistical Package for the Social Sciences (SPSS) Statistics, Version 25 (IBM Co., Armonk, NY) was used for all statistical analyses.
Results
A total of 60,701 urine cultures of 49,387 patients with a history suggestive of probable urinary tract infection were screened during the specified period (i.e., January 2017 to December 2021). Among 49,387 patients screened, 28,645 (58.1%) were females, whereas 20,742 (41.9%) were male. Among these 49,387 patients, 8125 urine cultures were determined with evidence of single microbial growth with a colony count of 105 colony-forming units/ml. Of 8125 patients diagnosed with UTI, 4754 were females and 3951 were males. Among 8125 patients with culture evidence of UTI, a total of 3106 patients had CR-UTI, 4184 patients had Carbapenem-sensitive UTI (CS-UTI) and 835 patients had Gram-positive UTI [Figure 1].
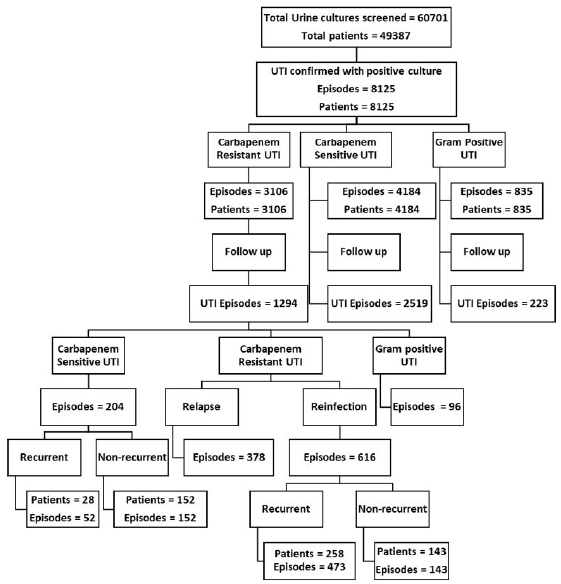
- Baseline flowchart of the study design, Urinary Tract Infection (UTI).
The study population was comprised of 1320 females and 1786 males. The mean age of the females and males was 52.86 years and 55.80 years, respectively. Among the study population, 1953 patients (62.9%) were diabetic, 1838 patients (59.2%) had a history of hypertension, 1012 patients (32.6%) had a history of ischemic heart disease, and 303 patients (9.7%) had a history of renal calculus. In addition, a total of 30 patients had a history of renal transplantation [Table 1].
| Characteristics | Present (n=3106) | Percentage | |
|---|---|---|---|
| Males | Number | 1786 | 57.50 |
| Mean age | 55.80 | ||
| SD | 21.40 | ||
| Females | Number | 1320 | 42.50 |
| Mean age | 52.86 | ||
| SD | 20.89 | ||
| Comorbidities | |||
| Diabetes mellitus | 1953 | 62.87 | |
| Hypertension | 1838 | 59.17 | |
| Ischemic heart disease/ Congestive heart failure | 1012 | 32.58 | |
| Peripheral vascular disease | 519 | 16.70 | |
| Cerebrovascular disease | 317 | 10.20 | |
| Renal calculi | 303 | 9.75 | |
| Malignancy | 274 | 8.82 | |
| Cirrhosis of liver | 208 | 6.69 | |
| Hypothyroidism | 176 | 5.67 | |
| Autoimmune disease on treatment | 99 | 3.18 | |
| Organ transplantation | 30 | 0.96 |
UTI: Urinary tract infection.
Among 3106 patients, a total of 752 patients (24.2%) had a history of broad-spectrum antibiotic administration in the last 30 days. Approximately, 23.2% (721) patients had a history of UTI in the last 6 months. A history of Carbapenem usage in the recent past (last 2 months) was noted in a total of 315 patients (10.1%). Additionally, other risk factors like history of urological interventions, surgery, mechanical ventilation, renal replacement therapy, and Double-J stent in situ were tabulated [Table 2].
| Risk factors | No of patients (n=3106) | Percentage |
|---|---|---|
| H/o Hospitalization with Intensive care unit admission in the last 2 months | 557 | 17.93 |
| H/o acute care and long-term care admission in the last 2 months | 694 | 22.34 |
| H/o Invasive mechanical ventilation | 376 | 12.11 |
| H/o Renal replacement therapy | 472 | 15.19 |
| H/o Surgery or urological procedures in the last 2 months | 394 | 12.68 |
| H/o Antibiotics before admission in the last 30 days | 752 | 24.21 |
| H/o Carbapenem usage in the last 2 months | 367 | 11.81 |
| H/o UTI episode in last 6 months | 721 | 23.21 |
| H/o Carbapenem-resistant UTI in last 6 months | 315 | 10.14 |
| Double J stent in situ | 438 | 14.10 |
| <4 weeks | 121 | 27.62 |
| 4 weeks–8 weeks | 189 | 43.15 |
| >8 weeks | 128 | 29.23 |
| Indwelling catheter in situ | 635 | 20.44 |
| >2 weeks | 197 | 31.02 |
| 2 weeks–4 weeks | 169 | 26.61 |
| >4 weeks | 269 | 42.37 |
H/O - History of.
Escherichia coli and Klebsiella pneumonia were the most common organisms, contributing to approximately 75% of episodes with CR-UTI [Figure 2]. Organisms like Pseudomonas and Acinetobacter species with predominant nosocomial UTI contributed to around 10% and 2%, respectively, of the total number of CR-UTI episodes [Figure S1].
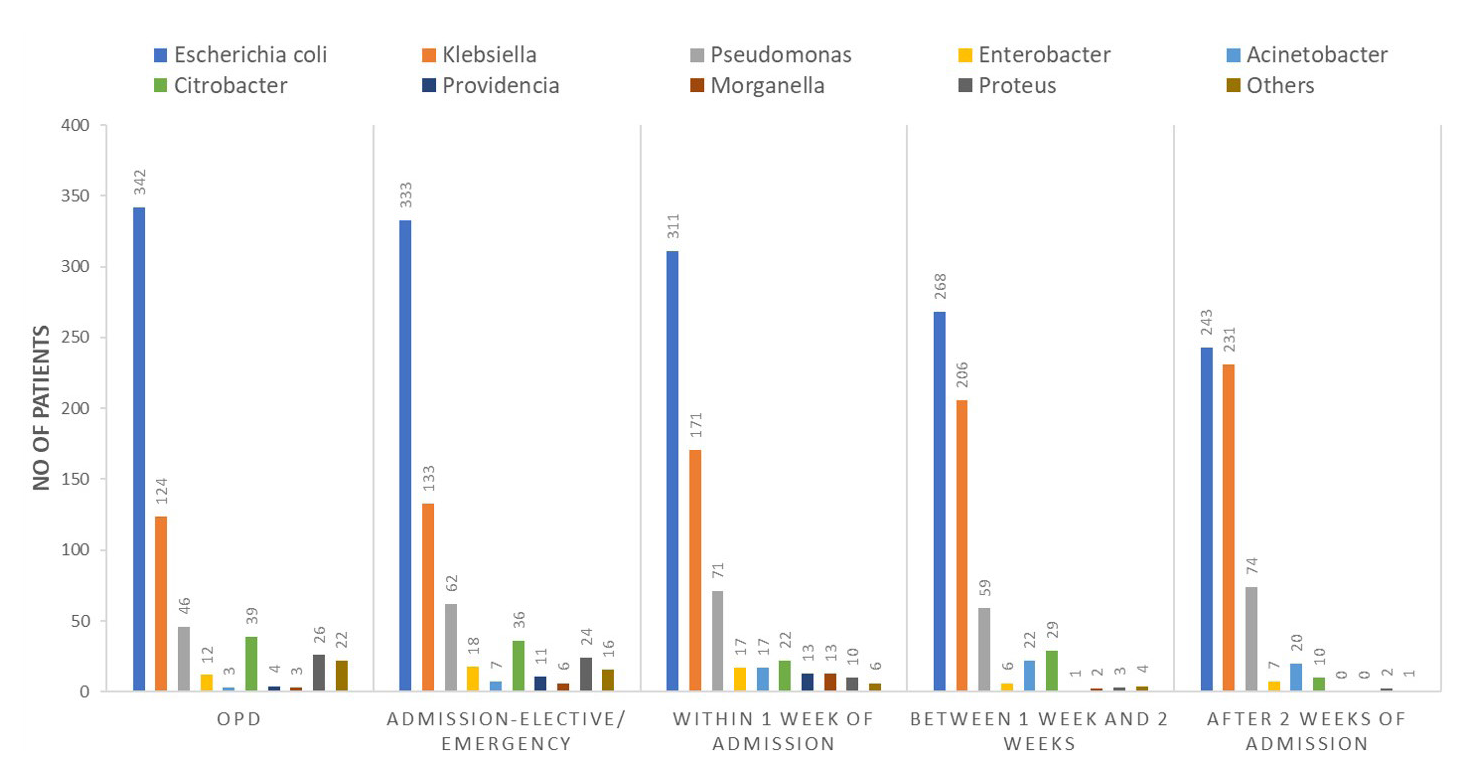
- Microbiological profile of organisms according to time of diagnosis of Carbapenem Resistant Urinary Tract Infection (CR-UTI). OPD: Out patient department.
Antibiotics resistance pattern
Among the bacteria causing CR-UTI, Carbapenem resistance among E. coli, Klebsiella species, Pseudomonas species, and Acinetobacter species was 80%, 90%, 90%, and 96%, respectively [Table S2].
Among 3106 patients, 2602 patients (83.77%) had evidence of renal dysfunction. Upon evaluation of these 2602 patients, a total of 1157 patients (44.47%) were found to have acute kidney injury, whereas the remaining 1445 patients (55.53%) were diagnosed with chronic kidney disease. Among 1157 patients who had AKI, 503 patients (43.37%) required hemodialysis among patients with AKI [Figure S2].
A total of 563 patients had either multiorgan dysfunction or septic shock with CR-UTI. Among these, approximately 67%, i.e., 377 patients had developed either multiorgan dysfunction or septic shock within 72 h of diagnosis. Among 3106 patients, 185 patients had evidence of bacteremia with the same organism during the episode of CR-UTI [Figure 3].
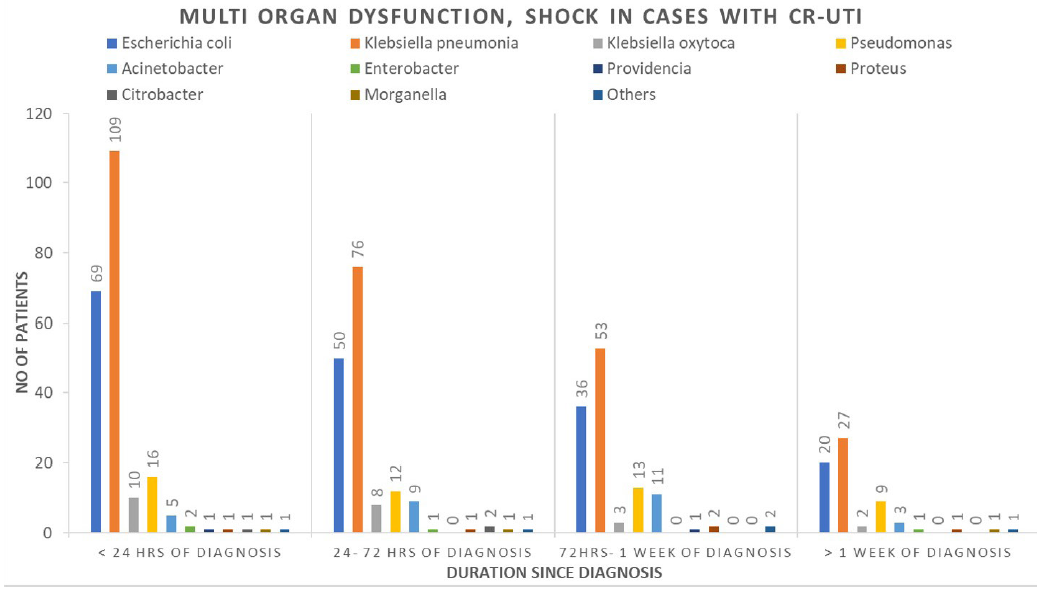
- Multiorgan dysfunction, septic shock in patients according to time of diagnosis of Carbapenem Resistant Urinary Tract Infection (CR-UTI).
Antibiotic treatment responses for E. coli, Klebsiella species, Pseudomonas species, Acinetobacter species, and other Gram-Negative species are depicted in Figures S3, S4, S5, S6, and S7, respectively.
The duration of antibiotics administered in patients with treatment response for CR-UTI caused by organisms of Enterobacteriaceae, Pseudomonas, and Acinetobacter species are depicted in Figures S8 and S9, respectively.
Of 3106 patients with CR-UTI, 380 patients (12.59%) had died within 28 days. Out of 380 deaths, 176 (46.32%) were within 24 h and 102 (26.84%) were between 24 and 72 h of diagnosis [Figure S10]. Kaplan–Meier survival analysis of patients with CR-UTI according to the individual organism was computed [Figure S11]. Patients with CR-UTI caused by Klebsiella oxytoca, Acinetobacter, and K. pneumonia were associated with inferior patient survival when compared to patients with CR-UTI caused by Pseudomonas species, E. coli, other Gram-negative Enterobacteriaceae.
The total number of patients under follow-up and patients noted to have UTI during follow-up at 1 month, at 2 months, at 3 months, at 6 months, and at 12 months were summarized [Figure S12]. A total of 1198 UTI episodes were noted among the patients who were under follow-up, out of which 994 episodes were CR-UTI and 204 episodes were CS-UTI (Carbapenem-sensitive). A total of 378 episodes (38%) of CR-UTI were due to relapses whereas the remaining 616 episodes were due to reinfection [Table S3].
Among 378 relapses, approximately 69%, i.e., 261 episodes were due to Klebsiella species, whereas 39 relapses were due to E. coli. Among 616 reinfection episodes with CR-UTI during follow-up, the predominant bacteria were K. pneumonia and E. coli, contributing to 47% and 24%, respectively [Figure S13].
A total of 77 patients (13.25%) had died during the follow-up. Patient survival at the end of 28 days in the recurrent and non-recurrent groups among both CS-UTI (85.78% vs 89.5%) and CR-UTI (85.81% vs 85.8%)) at follow-up were summarized with Kaplan–Meier survival analysis [Figure S14].
Discussion
In recent years, Carbapenem-hydrolyzing enzymes (carbapenemases) that confer drug resistance have emerged in K. pneumoniae and other Enterobacteriaceae.4,11-13 Although a study14 by Mohapatra et al. had shown that urinary pathogens causing community-acquired UTI had a prevalence of 52.8% of extended-spectrum beta-lactamase and 5.1% of Carbapenem-resistant isolates among E. coli, recent studies15-20 have noted increasing antimicrobial resistance, especially Carbapenem resistance ranging from 12.6% to 20.0%.
According to the latest Indian Council of Medical Research Antimicrobial Microbial Resistance Research and Surveillance Network Annual Report 2022,21 the Carbapenem susceptibility of E. coli, K. pneumonia, Acinetobacter, and Pseudomonas isolated from urine specimens of hospitalized patients was found to be 62.6%, 23.4%, 31.3%, and 26.2%, respectively. The prevalence of CR-UTI among UTIs caused by Gram-negative organisms in the current study is 31%, similar to previous studies.15-20
Various studies9,10 have noted that surgical procedures, dialysis, presence of an indwelling catheter, mechanical ventilation, poor functional status, admission to a long-term care facility, recent healthcare exposure, or hospitalization (especially an intensive care unit stay), and exposure to broad-spectrum antibiotics are risk factors for CRE colonization or infection. The current study also noted the presence of at least one risk factor among the patients with CR-UTI, with each risk factor attributing to an incidence of approximately 17%–25% of CR-UTI cases.
CR-UTIs contribute to approximately 31% of all Gram-negative UTIs. Escherichia coli and K. pneumonia are the most prevalent urinary pathogens, contributing to 75% of episodes of CR-UTIs. Most of the CR-UTIs diagnosed in the initial 72 h of admission were caused by either E. coli or Klebsiella species. However, if CR-UTI was diagnosed after 72 h of admission, the causative organisms were nosocomial bacteria which included predominantly Klebsiella species, Pseudomonas, and Acinetobacter.
Higher treatment response was seen with the use of Ceftazidime/Avibactam, Colistin, Ceftizoxime, Aminoglycoside, and Fosfomycin in patients of CR-UTI with organisms of Enterobacteriaceae for a minimum period of 7–10 days. At the same time, higher treatment response was seen with Fosfomycin, Aminoglycoside with Carbapenem, and Carbapenem with Colistin in patients with Pseudomonas or Acinetobacter species causing CR-UTI. Mortality is higher with patients having CR-UTI due to K. oxytoca, Acinetobacter, and K. pneumonia.
Most of the follow-up CR-UTI were within 3 months of which the majority are due to relapses. This might be due to inadequate duration of treatment or due to the persistence of modifiable risk factors. Reinfections were more common in K. pneumonia than in E. scherichia coli, possibly due to higher virulence. The 28-day mortality in the initial episode of CR-UTI is approximately 12%, whereas in patients with recurrent or non-recurrent episodes within a year is approximately 14%.
The current study is unique as it is a cohort study that included a large number of patients, whereas most of the prior studies were retrospective, cross-sectional studies. Other strengths of the study include analysis of the treatment response of every patient and consideration of follow-up of the patient for assessing relapse or reinfection. None of the prior studies have pragmatically analyzed various antibiotic treatment outcomes.
The current study did not compare the patient risk factors of CR-UTI with patients with CS-UTI. The current study only analyzed phenotypic analysis of organisms for Carbapenem resistance. Genotypic analysis of Carbapenem resistance would have helped us understand the various methods of transfer of Carbapenem resistance among the microorganisms and enlighten us in mitigating potential risk factors for the development of Carbapenem resistance.
Even with the use of efficient antibiotics, CR-UTIs were linked to early relapse and recurrence. It is imperative to address this rising pandemic with more modern, efficacious therapeutic, and preventive approaches.
Conflicts of interest
There are no conflicts of interest.
References
- Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of antibiotic sensitivity pattern of uropathogens in patients of different age groups from the western region of Nepal. Int J Med Res Health Sci. 2016;5:1-7.
- [Google Scholar]
- Recent sensitivity pattern of Escherichia coli in urinary tract infection. RRJMB. 2014;3
- [Google Scholar]
- Prevalence of urinary tract infections and current scenario of antibiotic susceptibility pattern of bacteria causing UTI. Indian J Microbiol Res. 2018;5:334-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and incidence of carbapenem-resistant K. pneumoniae colonization: Systematic review and meta-analysis. Syst Rev. 2022;11:240.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: Systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:668-77.
- [CrossRef] [PubMed] [Google Scholar]
- Carbapenem-resistant Enterobacteriaceae (CRE) infection: Clinician FAQs. CDC 2013 Mar 5
- [Google Scholar]
- Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect. 2012;18:439-48.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of illness in US hospitals due to carbapenem-resistant Gram-negative urinary tract infections in patients with or without bacteremia. BMC Infect Dis. 2021;21:572.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vital signs: Carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165-70.
- [PubMed] [PubMed Central] [Google Scholar]
- Carbapenemases in klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682-707.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Carbapenem-resistant enterobacteriaceae: A review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CAUTION-ED study (community-acquired UTI, emerging drug resistance). Antibiotic resistance of uropathogens among the community-dwelling pregnant and nonpregnant female: A step towards antibiotic stewardship. BMC Infect Dis. 2022;22:939.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Carbapenem resistance: A review. Med Sci (Basel). 2017;6:1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antimicrobial resistance in patients with suspected urinary tract infections in primary care in Assam, India. JAC Antimicrob Resist. 2021;3:dlab164.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of carbapenem-resistant escherichia coli and klebsiella pneumoniae in rural Uttar Pradesh. J Datta Meghe Inst Med Sci Univ. 2022;17:584-8.
- [CrossRef] [Google Scholar]
- Prevalence of carbapenem-resistant Enterobacteriaceae from a tertiary care hospital in Mumbai. India J Microbiol Infect Dis. 2013;3:207-10.
- [CrossRef] [Google Scholar]
- Phenotypic detection of carbapenemase-producing Gram-negative bacteria by modified Hodge test. Int J Curr Microbiol App Sci. 2016;5:315-20.
- [Google Scholar]
- Carbapenem-resistant Enterobacteriaceae: Prevalence and bacteriological profile in a tertiary teaching hospital from rural western India. Ind J Microbiol Res. 2018;5:342-7.
- [CrossRef] [Google Scholar]
- Indian council of medical research antimicrobial resistance research and surveillance network. annual report; 2022.








