Translate this page into:
Clinical Profile and Outcomes of Thrombotic Microangiopathy: A Kidney Biopsy Registry Cohort
Corresponding author: Selvin Sundar Raj Mani, Department of Nephrology, Christian Medical College Vellore, Ranipet Campus, Ranipet, Tami Nadu, India. E-mail: selvinsr@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Johny J, Alam R, Mani SSR, Jose N, Lalwani M, John EE, et al. Clinical Profile and Outcomes of Thrombotic Microangiopathy : A Kidney Biopsy Registry Cohort. Indian J Nephrol. doi: 10.25259/IJN_272_2024
Abstract
Background
Thrombotic microangiopathy (TMA) is caused by injury to microvasculature that leads to thrombus formation and multisystem dysfunction. TMA can cause irreversible kidney failure and graft failure in kidney transplant patients. The data on etiology, clinical and histopathological characteristics, treatment patterns, and renal outcomes of patients with TMA from resource-limited health care setups is less.
Materials and Methods
From a South Indian teritiary care center biopsy registry of 16,054 patients, 87 TMA cases diagnosed between January 2011 and April 2022 were included, and follow-up data were collected from electronic medical records until June 2023.
Results
The mean age of the cohort was 31.7± 9.9 years. The biopsy incidence of TMA was 0.5% during the study period. The most common TMA etiology was autoimmune disease (25.2%), followed by atypical HUS (18.4%) and pregnancy-associated and malignant hypertension (14.9%) each. The most common renal biopsy finding was mesangiolysis (74.7%), followed by capillary wall thrombi and fragmented RBCS. On a median 6-month follow-up (1,36), 24 (27.6%) patients showed renal recovery, and 40 (46%) remained dialysis-dependent. Multivariate analysis showed that dialysis dependence at presentation adversely affected renal recovery.
Conclusion
TMA, although rare, carries a high risk of renal failure and death. With early diagnosis and treatment, satisfactory renal outcomes can be achieved even in resource-limited health care settings.
Keywords
Biopsy
Microangiopathy
Plasma exchange
Thrombosis
Transplantation
Introduction
Thrombotic microangiopathy (TMA) is pathologically characterized by microvascular occlusion and clinically by thrombocytopenia and microangiopathic hemolytic anemia (MAHA).1 Its incidence is six in a million.2 The old classification of TMA into hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP) has changed following an improved understanding of the complement’s role in the TMA pathogenesis and the availability of genetic analysis.3 TMA etiology ranges from medication to genetic abnormalities. The propensity of the glomerular endothelium for microvascular injury makes the kidney prone to TMA. This condition’s impact is compounded by the fact that TMA affects younger people, including pregnant and postpartum females, often resulting in end stage kidney disease (ESKD). TMA is also an important cause of post-transplant graft dysfunction and failure. Some cases, like those caused by drugs can easily be treated by discontinuing the causative drug. Hence, the early diagnosis and treatment of this life and organ threatening disease is important. If recognized properly and treated timely, renal damage due to TMA can be minimized even in resource-limited health care settings. Data from developing countries on TMA etiology, demography, clinical presentation, histopathological features, and treatment outcomes are limited. We examined the important aspects of this often-misdiagnosed condition in adult patients, which will provide data specific to the resource-limited developing countries.
Materials and Methods
The study was approved by the institutional review and ethics committee (IRB No.15029 dated 06/01/2023). This observational retrospective study used the renal biopsy registry at the Christian Medical College Vellore in south India. In total, 16,054 adult kidney biopsies were performed between January 2011 and April 2022, of which 87 were diagnosed with TMA. Only renal biopsy (native and graft) proven cases in adult patients were included. Those patients with only hematological manifestations and no renal biopsy were excluded. TMA cases in children (<18 years) also were not included, as most of them are Shiga toxin-associated HUS and are not commonly biopsied. Snake bites, in tropical countries and TTP are relatively common causes of TMA. But these two entities were excluded as these cases are rarely biopsied.
Data on demographic profile, clinical features, histopathological variables, treatment details, and outcomes were retrieved from electronic patient records. The 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate the estimated glomerular filtration rate (eGFR).4 Since it is a retrospective study, waiver of consent has been taken from Institutional Ethics Committee.
All biopsy specimens were processed for light microscopy and immunofluorescence. Chronic TMA was defined by double contours of glomerular capillary walls, fibrous intimal thickening with onion skin-like lamination in the arteries, and hyalinosis in arterioles5 [Figure 1a]. Acute TMA was defined by the presence of thrombi, endothelial swelling or denudation, intramural fibrin, or intimal swelling in the arteriole [Figure 1b]. Thrombi, endothelial swelling, denudation, or mesangiolysis in the glomeruli were also classified as acute TMA [Figure 1c].
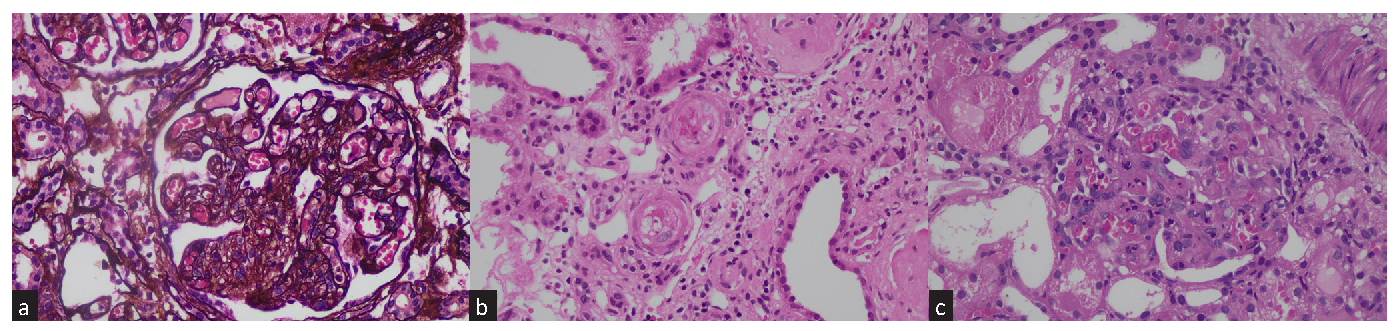
- (a) Chronic changes of TMA: Glomerulus shows global capillary wall reduplication along with concomitant endothelial swelling of capillary tuft obliteration, Jones Methenamine silver stain, X400. (b) Acute TMA changes in arteriole with fibrinoid necrosis, endothelial swelling, karyorrhectic nuclear debris, and luminal obliteration, H&E stain, X400. (c) Acute changes of TMA: Glomerulus shows segments of mesangiolysis, endothelial swelling, congestion and karyorrhectic nuclear debris. Adjacent tubules show acute injury with epithelial loss of brush border, epithelial simplification and cytoplasmic protein resorption droplets, H&E stain, X400. TMA: Thrombotic microangiopathy
The etiology of TMA was divided into several categories: pregnancy-associated (with normal ADAMTS 13, normal complement levels, and persistent renal dysfunction for > 6 weeks after delivery, thus requiring a renal biopsy), kidney transplant-related, post bone marrow transplant, lupus nephritis (LN), primary antiphospholipid antibody syndrome (APLA), scleroderma, malignant hypertension-related (presenting with diastolic BP >120 mmHg and hypertensive retinopathy features) and atypical HUS (with ADAMTS 13 activity >10%, no preceding diarrhea, features of MAHA, and no other etiologies or confirmation via genetic analysis).6 All renal-transplant cases were classified into transplant-related TMA.
Follow-up data were collected for each review visit until June 2023. Renal recovery was defined as eGFR >90 mL/min/1.73m2 or return to pre-morbid eGFR. Renal non-recovery was defined as failure to return to prebiopsy or eGFR < 15 mL/min/1.73m2 or kidney replacement therapy (KRT) initiation. For transplant outcomes, graft recovery was the return of eGFR to premorbid levels. Persistent graft dysfunction was defined as failure to return to premorbid eGFR level, and graft loss was defined as eGFR worsening to <15 mL/min/1.73 m2 for > 3 months OR initiation of dialysis. For pregnancy outcomes, low birth weight was defined as < 2500 g irrespective of gestational age (WHO). Hematological recovery was defined as platelet >150000/mm3 and no lab parameters suggestive of active hemolysis, like schistocytes, hemoglobin drop, reticulocytosis, or LDH level > 500 U/L.
Statistical analysis
All clinical, laboratory, and histological data were entered in the Epidata version 4.6.0.6, and analysis was done using SPSS software version 25. Baseline characteristics were reported as mean ± standard deviation for normally distributed quantitative variables and median (IQR) for skewed variables. Categorical data were expressed as number (%). Differences among normally distributed variable groups were analyzed by the student t test. Differences among groups of nonparametric variables were analyzed by the Mann–Whitney U or Kruskal–Wallis tests. Categoric variables were compared using Pearson’s chi-squared or Fisher’s exact tests. Univariate and multivariate analyses for renal non recovery predictors were done using the Cox proportional hazard regression method.
Results
A total of 87 patients (0.5% of all biopsies) were included. Eleven (12.6%) were graft biopsies and 76 were native kidney biopsies. The mean ± (SD) age of patients was 31.7 ± (9.9) years. Females constituted 57.5% of the study population. At the time of clinical diagnosis, there were 16 pregnant or postpartum patients. Of these, three were diagnosed with primary APLA, classified as APLA-associated TMA. There were 19 post-transplant patients (21.8%), of which 11 were post-kidney transplant (KT), and 8 were post-bone marrow transplant patients (BMT). All 11 KT patients were on calcineurin Inhibitors (CNI) at the time of TMA diagnosis and were classified as transplant-associated TMA. There were 22 (25.2%) patients with underlying autoimmune disease; LN was the most common. Edema was the most common symptom in 52 (59.8%) patients, followed by oliguria in 48 (55.2%). The other baseline characteristics have been summarized in Table 1.
| Baseline characteristics at biopsy | Entire cohort (n=87) |
|---|---|
| Age (years) | 31.7 ± 9.99 |
| Sex | |
| Female | 50 (57.5%) |
| Comorbid illness | |
| Chronic kidney disease | 11 (12.6%) |
| Diabetes mellitus | 4 (4.6%) |
| Etiology | |
| a-HUS | 16 (18.4%) |
| LN | 15 (17.2%) |
| Malignant hypertension | 13 (14.9%) |
| Pregnancy | 13 (14.9%) |
| Post kidney transplant | 11 (12.6%) |
| Post bone marrow transplant | 8 (9.1%) |
| Primary APLA | 5 (5.7%) |
| Scleroderma | 2 (2.3%) |
| Drugs (Gemcitabine) | 1 (1.1%) |
| Others* | 3 (3.6%) |
| Transplant related TMA (n =19) | |
| Kidney | 11 (57.9%) |
| Bone marrow | 8 (42.1%) |
| Type of autoimmune diseases (n =22) | |
| SLE | 15 (68.1%) |
| APLA | 5 (22.7%) |
| Scleroderma | 2 (9.0%) |
| Clinical features | |
| Edema | 52 (59.8%) |
| Oliguria | 48 (55.2%) |
| Fever | 17 (19.5%) |
| Macroscopic hematuria | 4 (4.6%) |
| Systolic BP (mmHg) | 148.8 ± 28.6 |
| Diastolic BP (mmHg) | 92.8 ± 19.2 |
Autoimmune disease-associated TMA was the most common (25.2%). This included LN, scleroderma, and APLA-associated TMAs. It was followed by a-HUS (18.4%) and malignant hypertension (14.9%). One patient (heterozygous mutation for CFH1) with kidney failure due to a-HUS in the native kidneys developed graft failure 1-month post-transplant. The graft biopsy showed cortical necrosis features. Other causes have been described in Table 1.
The mean hemoglobin (SD) at presentation was 8.1 ± 1.9 g/dL, and the median platelet count (IQR) was 99,000 (58,000-1,73,000)/mm3. Microscopic hematuria was present in 61 patients (70.1%). Median eGFR (IQR) at biopsy was 13.4 mL/min/1.73m2 (7.2,41.5). At the time of kidney biopsy, 54 (62.1%) patients were dialysis dependent. Acute TMA changes were observed in 71 (81.6%) patients. Capillary thrombi were seen in 62 (71.3%) patients and arterial thrombi in 38 (43.7%). Crescents were observed in seven (8%) patients, of which four were fibro-cellular, two were cellular, and one was fibrous crescent. Arteriosclerosis of various degrees was seen in 71 (81.60%) biopsies [Table 2]. Renal-limited TMA was observed in 25 (28.7%) patients, and 62 (71.3%) had renal and hematological TMA manifestations [Table 3].
| Laboratory parameter | Entire cohort (n=87) |
|---|---|
| Hemoglobin (g/dL) | 8.1 ± 1.9 |
| Platelet count (cells/mm3) | 99000 (58000,173000) |
| Albumin (g/dL) | 3.18 ± 0.76 |
| LDH at presentation (U/L) | 911 (623.5, 1596.5) |
| Percentage of schistocyte | 0.85 (0.1,2) |
| Reticulocyte count (%) | 2.69 (1.75, 5.37) |
| Creatinine at presentation (mg/dL) | 4.85 (2.1, 7.5) |
| eGFR at biopsy (mL/min/1.73m2) | 13.4 (7.2, 41.5) |
| Dialysis dependent at presentation | 54 (62.1%) |
| 24-hour urine protein at presentation (mg/24 hours) | 1144 (343.2, 2607.2) |
| Microscopic hematuria | 61 (70.1%) |
| PT INR within normal range | 81 (93.1%) |
| Normal APTT (sec) | 75 (86.2%) |
| C3 level (Available in 78 patients) (mg/dL) | |
| >100 | 36 (46.2%) |
| 75-100 | 21 (26.9%) |
| 50-75 | 7 (8.9%) |
| <50 | 14 (17.9%) |
| Number of glomeruli | 13.69 ± 5.98 |
| Mesangiolysis | 65 (74.7%) |
| Capillary thrombi | 62 (71.3%) |
| Fragmented RBCs | 62 (71.3%) |
| GBM thickening | 60 (69%) |
| Mesangial expansion | 60 (69%) |
| Capillary wall wrinkling | 57 (65.5%) |
| Tuft obliteration | 51 (58.6%) |
| Endothelial swelling | 55 (63.2%) |
| Fibrinoid necrosis | 40 (46%) |
| GBM duplication | 34 (39.1%) |
| Presence of crescents | 7 (8%) |
| Fibro cellular | 4 |
| Cellular | 2 |
| Fibrous | 1 |
| Presence of acute tubular necrosis | 72 (82.8%) |
| Severity of arteriosclerosis | |
| None | 16 (18.4%) |
| Mild | 52 (59.8%) |
| Moderate | 15 (17.2%) |
| Severe | 4 (4.6%) |
| Presence of arterial thrombosis | 38 (43.7%) |
| Immunofluorescence | |
| Negative | 53 (60.9%) |
| Full house | 16 (18.4%) |
| IgG & C3 | 6 (6.8%) |
| IgM, IgG, IgA& C3 | 4 (4.6%) |
| C3 alone | 3 (3.4%) |
| IgM alone | 3 (3.4%) |
| IgM and C3 | 2 (2.3%) |
| Type of TMA on renal biopsy | |
| Acute | 71 (81.6%) |
| Chronic | 16 (18.4%) |
APTT: Activated partial thromboplastin time, eGFR: estimated glomerular filtration rate, LDH: Lactate dehydrogenase, PT INR: Prothrombin time/International normalised ratio, GBM: Glomerular basement membrane, IQR: Interquartile range, LDH: Lactate dehydrogenase, RBC: Red blood cells, TMA: Thrombotic microangiopathy
| Platelet transfusions | 12 (13.8%) |
|---|---|
| PLEX required | 57 (70.4%) |
| Dialysis requirement at biopsy | 54 (62.1%) |
| Type of treatment received | |
| Supportive treatment and antihypertensives | 16 (18.4%) |
| Plasma infusion | 2 (2.3%) |
| Plasma infusion and PLEX | 11 (12.6%) |
| Prednisolone alone | 9 (10.3%) |
| Prednisolone and PLEX | 24 (27.5%) |
| Rituximab alone | 1 (1.1%) |
| Rituximab and PLEX | 6 (6.9%) |
| Rituximab and Prednisolone | 2 (2.3%) |
| Prednisolone, MMF, and PLEX | 7 (8.0%) |
| Prednisolone, Cyclophosphamide, and PLEX | 9 (10.3%) |
| Eculizumab | 0 |
| Renal function (eGFR) at different timelines (mL/min/1.73m2) | |
| At Discharge (n=81) | 17.2 (8.7, 49) |
| 1 month (n=36) | 37.6 (18.9,69.4) |
| 3 months (n=28) | 49.1 (34.2, 71.2) |
| 6 months (n=31) | 50.5 (33.5, 68) |
| 1 year (n=27) | 52.1 (31.7, 73.8) |
| Dialysis dependence at the last follow up | 40 (46%) |
| Hemoglobin at different timelines (g%) | |
| Discharge (n=81) | 8.9 ± 1.5 |
| 1 month (n=36) | 9.8 ± 2.2 |
| 3 months (n=28) | 10.6 ± 2.3 |
| 6 months (n=31) | 11.3 ± 2.6 |
| 1 year (n=27) | 11.7 ± 2.1 |
| Last follow up outcome | |
| Renal limited TMA | 25 (28.7%) |
| Renal recovery | 9 (36%) |
| No renal recovery | 15 (60%) |
| Death | 1 (4%) |
| TMA with renal and hematological manifestations | 62 (71.3%) |
| Hematological recovery alone | 27 (43.5%) |
| Renal recovery alone | 0 |
| Both hematological or renal recovery | 15 (24.2%) |
| No hematological or renal recovery | 12 (19.4%) |
| Death | 8 (12.9%) |
| If pregnant – outcome of pregnancy (n=16) | |
| Term delivery with normal birth weight | 3 (18.8%) |
| Low birth weight | 4 (25.0%) |
| Stillbirth | 6 (37.5%) |
| Neonatal death | 3 (18.8%) |
| If renal transplant – graft outcome (n=11) | |
| Graft recovery | 6 (54.5%) |
| Graft loss | 2 (18.2%) |
| Persisting graft dysfunction | 1 (9.0%) |
| Death with functioning graft | 2 (18.2%) |
eGFR: estimated glomerular filtration rate, IQR: Interquartile range, MMF: Mycophenolate mofetil, PLEX: Plasma exchange, TMA: Thrombotic microangiopathy
Sixteen (18.4%) patients received only supportive treatment, like initiation of anti-hypertensives to control BP and KRT. Plasma exchange (PLEX) and immunosuppressive medications were given to 57 (65.5%) and 58 (66.6%) patients, respectively [Table 3]. Infection risk increased in those receiving PLEX/immunosuppression. Of the 57 patients receiving PLEX, 27 had severe infections like catheter-related bloodstream infections (most common), urinary tract infections, renal abscesses, graft pyelonephritis, and pneumonia.
Outcomes
At discharge, only 23 (26.4%) patients had both hematological and renal recovery. Six (6.9%) died during the hospital stay. On the median 6-month follow-up (1,36 months), nine (36%) patients with renal-limited TMA had renal recovery, and 15 (24.2%) with both renal and hematological involvement had renal recovery depicting an overall poor renal outcome [Supplemental Table 1]. There were 40 (46%) dialysis-dependent patients on the last follow-up. The median (IQR) eGFR at time of discharge was 17.2 (8.7, 49) mL/min/1.73m2 [Table 3]. Patients receiving plasmapheresis showed statistically significant dialysis dependence over those who did not (OR, 3.6; 95% CI, 1.42-9.3). There was no significant renal outcome between those who had acute and chronic changes on biopsy (The unadjusted HR, 1.32; 95% CI, 0.69-2.51).
The fetal outcome was not favorable for pregnancies complicated by TMA. Only three (18.8%) out of 16 pregnancies complicated by TMA had full-term normal deliveries. Six (37.5%) resulted in stillbirths, and four (25.0%) infants had low birth weight.
The mortality rate was 10.3%, with six patients dying during the first hospital admission and three patients during follow-up.
Graft outcome
The median (IQR) kidney transplant vintage at TMA diagnosis was 38 days (5,95). All KT patients with TMA were on tacrolimus. One graft biopsy showed definitive CNI toxicity features, whereas 5 showed antibody mediated rejection (ABMR) features. All transplant patients received PLEX. Two patients died due to treatment complications, one due to an intracranial bleed, and one due to disseminated intracranial coagulation (DIC). Details of post-renal transplant TMA have been given in Supplemental Table 2.
Eight post- BMT patients developed TMA during the study period. None of the patients were on drugs capable of causing TMA at the time of diagnosis. Four patients had a history of non-renal Graft Versus Host Disease and were on treatment for the same. Except for two patients with severe renal failure at TMA diagnosis, all others were treated with prednisolone +/ MMF or rituximab. Three patients received PLEX. On follow-up, two (25%) patients had normal renal function, while one (12.5%) had progressed to ESKD, and four (50%) had persistent renal dysfunction. One patient died during the hospital stay due to multiple infections following PLEX, prednisolone, and rituximab.
Univariate analysis found male gender, arterial thrombosis, and dialysis requirement at biopsy as factors significantly affecting renal recovery. Multivariate analysis found dialysis requirement at biopsy as the only statistically significant factor that adversely affected renal recovery [Supplemental Table 1].
Discussion
TMA is a spectrum of disorders characterized by microvasculature injury, causing endothelial damage and thrombi formation, resulting in organ injury. TTP was first described by Moschcowitz in 1924, in a teenage girl. HUS was described by Gasser in 1955.7 We showed multiple TMA etiologies that cannot always be classified into TTP or HUS alone, as done previously. This study shows that TMA mainly affects the young (mean age was 31.7 years). There were >55% females, probably because of TMA’s association with pregnancy and autoimmune diseases, which are common in young females. The study also highlights the severity of renal failure due to TMA, as the median GFR was <15 mL/min/1.73m2, and > 60% of patients were on dialysis at the time of biopsy.
A retrospective study from India found malignant hypertension followed by post-partum TMA to be the most common etiologies of TMA.8 We found autoimmune disease-related TMA to be the most common (>1/4 of our cases). LN was the leading autoimmune disease-causing TMA. Some studies have found the TMA proportion in LN patients to be as high as 25%.9
Pregnancy accounts for 8-18% of TMA cases.10 This study corroborates these findings, with ∼15% of cases being associated with pregnancy. The increased TMA risk in pregnancy is due to increased procoagulant factors, decreasing fibrinolytic activity, loss of endothelial cell thrombomodulin, and decreasing ADAMTS13 activity.11 Pre-eclampsia, DIC, and post-partum hemorrhage, can predispose females to TMA.12 The pregnancy outcome in our cohort was not favorable; only three patients had normal-term delivery. The rest of the cases were complicated with stillbirth, neonatal death, or low birth weight infants.
The TMA incidence in renal transplant patients varies between 0.8-14%.13 It can be de novo or recurrence of the native kidney disease. The TMA risk is highest in the first three months after transplant.14. A retrospective study from India showed acute rejection as the most common cause of post-renal transplant TMA, followed by CNIs.15 TMA in the graft can be a manifestation of CNI toxicity or rejection, as shown in five patient biopsies. Overall post-kidney transplant prognosis was poor, with graft loss in up to 40% of the cases in the first 2 years, similar to our study.
Renal biopsy findings can be varied in TMA. Glomerular or arteriolar thrombi are pathognomonic.16 Mesangiolysis, the presence of fragmented RBCs capillary thrombi, and endothelial swelling were the most common features in acute TMA. Glomerular basement membrane (GBM) thickening and duplication were observed in chronic cases. Arterial thrombosis was found to be higher than in similar studies, probably because of the higher number of the etiologies causing arterial injury like malignant hypertension, aHUS, scleroderma, and APLA-related TMA. Very importantly, ∼20% of our cases had chronic biopsy changes although the symptom duration was short [Supplemental Table 3].
Treatment depends on the etiology. However, in a case with TMA diagnosis and no immediate evident cause, it is important to quickly initiate PLEX after taking samples for ADAMTS13, as TTP prognosis without PLEX is very poor.17 In our cohort >70% of patients received PLEX. PLEX results in the administration of high doses of complement-regulating proteins, removal of dysfunctional endogenous soluble complement inhibitors and anti-FH antibodies.18 Eculizumab the complement inhibitor were shown to improve the renal prognosis in patients with CM-TMA.19
The treatment in this study involved withholding the possible offending drug in case of drug-induced TMA, and control of hypertension in malignant hypertension-associated TMA, in addition to the other supportive treatment. Immunosuppression (IMS) was given based on the etiology, e.g., induction of remission in LN. In those with acute changes on biopsy and persistent renal dysfunction after PLEX, IMS with steroids or concomitantly with rituximab or cyclophosphamide were used. Those with chronic changes on biopsy and dialysis dependence after treatment with antihypertensives and PLEX were continued on non-immunosuppressive therapy, including dialysis. None of the patients received eculizumab due to non-availability. Renal outcomes in TMA remain poor despite advances in diagnosis and treatment. Patients are at high-risk of long-term complications like hypertension, stroke, pre-eclampsia, CKD, and cognitive impairment.20 Although our study showed higher dialysis dependence in those who received PLEX, it may be due to the fact that those receiving plasmapheresis had more severe disease at presentation.
At presentation, >60% of our patients were dialysis dependent, highlighting the condition’s severity. On the last follow-up ∼50% of our patients remained dialysis-dependent. Dialysis dependence at biopsy was significantly associated with poor renal outcomes. The TMA prognosis in renal transplants is poor and the recurrence rate is between 60-70%.17 One patient had TMA recurrence within 3 months post-renal transplant. The study also showed hematological recovery of ∼70% and renal recovery in < 30% of cases. The mortality rate was high (10%), comparable to a similar study by Yu et al.21 [Table 4].
| Study | Yu et al.21 | Manickam et al.8 | Bayer et al.6 | Present study |
|---|---|---|---|---|
| Country/Region | China | India | France | India |
| Study duration (years) | 2000-2012 (12) | 2012-2017 (5.5) | 2009-2016 (8) | 2011 -2022 (11.5) |
| Study design | Retrospective | Retrospective | Retrospective | Retrospective |
| Total number of cases | 109 | 40 | 564 | 87 |
| Mean age of participants (years) | 34.0 ± 11.1 | 31±12 | 37 | 31.7 ± 9.9 |
| Follow up duration (months) | 30.5 ± 36.5 | 8.8 ± 13 | 6 (1,36) | |
| Most common etiology | Malignant hypertension (56%) | Malignant hypertension (39%) | Pregnancy (35%) | Autoimmune (25.2%) |
| Proportion of patients receiving PLEX | 13.8% | 45.5% | 16% | 65.5% |
| Most common IMS received by patients | Steroids | Steroids | Steroids | |
| Persistent renal dysfunction or ESRD on last follow up | 56% | 20% (On dialysis) | 72.4% | |
| Mortality | 7.3% | 10% | 10.3% | |
| Statistically significant factors causing poor renal outcome | Male gender, Elevated serum creatinine Low Hb Presence of chronic renal TMA pathologic changes | Male gender, Arterial thrombosis Dialysis requirement at biopsy |
ESRD: End stage renal disease, Hb: Hemoglobin, IMS: Immunosuppressant, PLEX: Plasma exchange, TMA: Thrombotic microangiopathy
In conclusion, our study highlights the significant morbidity and mortality in TMA irrespective of etiology, especially in developing countries. The study also shows condition’s predilection for the young population. TMA can complicate autoimmune diseases, which were the most common etiology, renal and BM transplants, and pregnancy. Thus, it is important to remember this differential diagnosis when these patients present with new onset or worsening renal or hematological parameters. Timely diagnosis and appropriate treatment increase the chance of renal and patient recovery. There is an urgent need for newer diagnostic modalities like complement studies and genetic analysis, as well as the availability of treatment options like complement inhibitors in developing countries. Our study is novel from a developing country with a large cohort who had biopsy-proven diagnoses.
This was a single-center study. Genetic analysis and complement factor analysis were done in only a few patients due to logistic reasons. Hence and some cases classified as transplant, pregnancy, or hypertension-related cases may have had underlying complement/genetic abnormalities. However, it reflects the real-life scenario of a clinical set-up in a developing world. CFIs like eculizumab were not given to any patient.
Acknowledgements
We acknowledge the contribution of Dr. Pippa Deodar (Scientist at the Principal’s office) in the proof reading of the manuscript. We also acknowledge the contribution of Miss Jansi Rani and Dr. Midhun S in the statistical analysis of the results.
Conflicts of interest
There are no conflicts of interest.
References
- Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol.. 2008;3:249-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323-35.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol. 2018;13:300.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A new pathological perspective on thrombotic microangiopathy. Kidney Res Clin Pract. 2022;41:524-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Etiology and outcomes of thrombotic microangiopathies. Clin J Am Soc Nephrol. 2019;14:557-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Natural history of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Semin Thromb Hemost. 2014;40:866-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-Histological features of thrombotic microangiopathy in renal biopsies: A retrospective study. Turk Patoloji Derg. ;38:1-8.
- [CrossRef] [Google Scholar]
- Effect of thrombotic microangiopathy on clinical outcomes in Indian patients with lupus nephritis. Kidney Int Rep. 2017;2:844-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol. 2012;7:2100.
- [CrossRef] [PubMed] [Google Scholar]
- The association of pregnancy with thrombotic thrombocytopenic purpura–hemolytic uremic syndrome. Curr Opin Hematol. 2003;10:339-44.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum hemorrhagic shock resulting in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome. J Matern-Fetal Neonatal Med. 2003;13:208-10.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic microangiopathy after kidney transplantation: An underdiagnosed and potentially reversible entity. Frontiers in medicine [Internet]. 2021 [cited 2024 Jan 7];8 Available from: https://www.frontiersin.org/articles/10.3389/fmed.2021.642864
- [Google Scholar]
- Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003;42:1058-68.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic microangiopathies postrenal transplantation. Indian J Transplant. 2020;14:213.
- [CrossRef] [Google Scholar]
- Renal thrombotic microangiopathy: A review. Am J Kidney Dis. 2023;81:591-605.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of thrombotic microangiopathy. Int J Lab Hematol. 2022;44(Suppl 1):101-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia [Internet]. 2015 [cited 2024 Jan 7];35(5) Available from: https://pubmed.ncbi.nlm.nih.gov/26456110/
- [CrossRef] [PubMed] [Google Scholar]
- Terminal complement inhibitor eculizumab in atypical hemolytic–Uremic syndrome. N Engl J Med. 2013;368:2169-81.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes of thrombotic microangiopathy treated with plasma exchange: A systematic review. Am J Hematol. 2016;91:623-30.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and renal biopsy findings predicting outcome in renal thrombotic microangiopathy: A large cohort study from a single institute in China. The Scientific World Journal. 2014;2014:e680502.
- [CrossRef] [Google Scholar]








