Translate this page into:
The Clinical Profile and Long-Term Outcome of Children with Membranous Nephropathy, and the Evaluation of Anti-Phospholipase A2 Receptor Antibody Immunohistochemistry in Kidney Biopsy
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Idiopathic membranous nephropathy (iMN) is a rare cause of nephrotic syndrome in children (1%–7%). Anti-phospholipase A2 receptor (PLA2R) antibody positivity in kidney biopsy is observed in 52%–78% of adults and 45% of children with iMN. The objectives of the study are to analyze the clinical profile and outcome of membranous nephropathy in children, to assess the prevalence of anti-PLA2R immunohistochemistry (IHC) in kidney biopsy, and to correlate their presence with disease characteristics.
Methods:
We are reporting a single-center retrospective study conducted in pediatric nephrology division. Clinical data and outcome parameters of children with membranous nephropathy were analyzed. PLA2R IHC was performed in kidney biopsy specimens retrospectively.
Results:
We analyzed 43 children with membranous nephropathy (MN) from a single center. 18 (42%) had idiopathic MN (iMN). PLA2R IHC was performed in kidney biopsy specimens in 14/18 (78%) patients with iMN and 7/9 (78%) non-lupus secondary membranous nephropathy (SMN) patients. The most common cause of SMN was lupus nephritis in 16 patients (64%). The mean estimated glomerular filtration rate (eGFR) at onset was 156 ± 81 ml/min/1.73m2. The sensitivity and specificity of PLA2R IHC in diagnosing pediatric MN was 50% and 57%, respectively; positive and negative predictive values were 70% and 36%, respectively. At the final follow-up, chronic kidney disease stage 5 (CKD 5) developed in 2/14 (14.3%) iMN patients.
Conclusions:
IHC PLA2R staining of glomerular tissue is a useful diagnostic marker of IMN. Though PLA2R prevalence is lower in children, its role in guiding treatment needs further exploration.
Keywords
Anti-phospholipase A2 receptor antibody (PLA2R)
children
immunohistochemistry
kidney biopsy
membranous nephropathy
Introduction
Idiopathic membranous nephropathy (iMN) is a rare cause of nephrotic syndrome (NS) in children. M-type phospholipase A2 receptor antibody (PLA2R) positivity in kidney biopsy is observed in adults, with a sensitivity of 52%–78%.[1-4] It varies in children from 6% to 83%.[5,6] Its demonstration is important from the treatment perspective, as it aids in decision regarding immunosuppressive therapy, follow-up, and prognosis.
Most iMN is mediated by antibodies to the PLA2R (85%), thrombospondin type 1 domain-containing 7A (THSD7A) (3%–5%),[7] or by yet unidentified mechanisms (10%). The new antigens discovered are exostosin 1/2 (EXT1/2), neural epidermal growth-like 1 protein (NELL1), semaphorin 3B (SEMA3B),[8,9] protocadherin 7 (PCDH7), and neural cell adhesion molecule 1 (NCAM1).[10,11] SEMA3B is observed to have a pediatric predominance. The treatment of iMN utilizes immunosuppressive regimens, whereas the treatment of secondary membranous nephropathy (SMN) is targeted at the underlying primary etiology. The prevalence of circulating and tissue PLA2R were reported in varied proportions from different regions.[12-16] PLA2R staining in kidney biopsy persists for months after the antibody disappears in serum.[17] In contrast to adults, children have higher overall remission rates (75%) and lower incidence of impaired kidney function.[18] Lee et al.[19] reported 18% progression to chronic kidney disease (CKD) in children in comparison to 30%–35% progression in adults.
The commonly utilized immunosuppressive regimens lead to marked reduction in circulating PLA2R within 3–4 months, followed by disappearance of antibody within 6–9 months, and remission of proteinuria in 12–24 months in more than 80% iMN cases. Recent serology-based approach for management of iMN has been guided by the initial and follow-up serum anti-PLA2R antibody levels.[20,21]
In this study, we analyzed the clinical profile and outcome of membranous nephropathy (MN) in children, assess the prevalence of PLA2R positivity in kidney biopsy by immunohistochemistry (IHC) and to correlate it with the clinical severity and long-term outcome.
Materials and Methods
Patient selection
This 15-year retrospective study was conducted in the pediatric nephrology division at a tertiary care hospital in South India. Children below 16 years who were diagnosed as MN by kidney biopsy from Jan 2005 to Oct 2020 were included. Anti PLA2R IHC was performed in the archived kidney biopsy specimens. The objective was to evaluate the clinical profile and long-term outcome of both iMN and SMN and assess the sensitivity of PLA2R IHC in children with iMN. Institutional research board and ethical committee clearance was obtained. Waiver of consent was sought since retrospective analyses of archived renal biopsy specimens were performed and data were collected from electronic health records.
Clinical details with definitions
Demographic and biochemical data were collected from electronic records and collected at onset, quarterly till 12 months, and thereafter at 2, 5, and 10 years. Secondary causes of MN were ruled out by performing hepatitis B virus surface antigen (HBsAg), human immunodeficiency virus (HIV), hepatitis C, anti-nuclear antibody, chest X-ray, ultrasound abdomen, and stool occult blood in necessary cases.
NS was defined as per the kidney disease: improving global outcomes (KDIGO) guidelines 2012.[22] The Toronto risk score is not feasible in children since all childhood-onset NS cases receive corticosteroids before kidney biopsy unlike adult NS.[23] Hypertension (HTN) was defined as blood pressure above 95th percentile for age, gender, and height as per the Fourth Report.[24] Estimated glomerular filtration rate (eGFR) was obtained using the modified Schwartz’s formula.[25] Microscopic hematuria was defined as more than five red blood cells (RBCs) per high-power field of centrifuged urine sample.
Complete remission (CR) was defined as urine protein nil or trace and urine protein creatinine ratio (uPCR) <0.3 mg/mg with a normal serum albumin and creatinine; partial remission (PR) was defined as uPCR <3.5 mg/mg and a 50% or greater reduction from baseline with a stable eGFR and serum albumin >3.5 g/dL. Nonresponse was lack of CR or PR after 6 months of therapy. Relapse was defined as recurrence of proteinuria after being in remission previously.[26,27] The choice of the immunosuppressive agents was as per the discretion of the treating physician and was based on patient characteristics, previous treatment, side effect profile, affordability of drugs, and parental preference.
Histopathology
Kidney biopsy samples were fixed in 10% neutral buffered formalin and embedded using standard methods. Tissue for light microscopy consisted of serial 3-μm-thick sections, stained with hematoxylin and eosin, periodic Schiff reagent, Masson trichrome, and Jones methanamine silver stains. Immunofluorescence studies consisted of 4 μm cryostat sections stained with fluorescein-tagged polyclonal rabbit anti-human antisera specific to IgG, IgA, IgM, complement factors C3, C1q, and to kappa and lambda light chains (all from Dako, Carpinteria, CA, USA).
IHC staining for anti-PLA2R antibody (Sigma Aldrich) was performed on “Ventana Benchmark XT autostainer” multimer method with diaminobenzidine. Dilution of the primary antibody used was 1:1000. Positive IHC staining of anti-PLA2R antibody was defined by the complete staining of the glomerular capillaries. All our cases showed either strong PLA2R positive or absent staining of glomerular capillary walls, as shown in Figure 1.

- (a) Granular staining for PLA2R1 along glomerular basement membranes in a patient with idiopathic membranous glomerulopathy (immunohistochemistry; original magnification 400). (b) Glomerulus from patient with idiopathic membranous glomerulopathy is completely negative for PLA2R1 (immunohistochemistry; original magnification 400).
Statistical analysis
Data were presented as the mean ± standard deviation or median (interquartile range [IQR]) depending on the normality of distribution. Student’s t-test and analysis of variance (ANOVA) were used for parametric analysis. Comparison of means was done using independent samples t-test. Correlation between two parameters was assessed using Pearson coefficient. Statistical significance was considered when P < 0.05. Statistical analysis was done using IBM Statistical Package for the Social Sciences (SPSS) Statistics Version 21 (IBM Corp., Armonk, NY, USA)
Results
Patient characteristics
MN constituted 3.2% of pediatric kidney biopsies over 15 years. They were further divided into iMN in 42% (n = 18) and SMN in 58% (n = 25). Figure 2 depicts the flow diagram of the study indicating enrolment and follow-up. In the study group, the incidence of MN increased as age progressed, with 7% of MN in 1–5 years, 23% in 6–10 years, and 70% in 11–15 years. In children below 10 years of age, SMN was significantly more than iMN (69%, P = 0.04). At the initial presentation, nephrotic proteinuria was present in 90.7% (n = 39) with uPCR of 6.9 (3.4, 11.2), microhematuria in 95% (n = 41), and hypertesion in 25% (n = 11) of cases. The mean eGFR at the onset was 156 ± 81 ml/min/1.73m2. NS was present in 54% (n = 23/43) patients. The demographic features are presented in Table 1. The presentation in childhood iMN was late-onset NS and steroid-resistant NS in eight patients each (44.4%) and nephritic-onset NS in two (11%) patients. Most common causes of SMN were lupus nephritis (16, 64%), hepatitis B (8%), infection-related glomerulonephritis (8%), non-lupus full house nephropathy (8%), graft versus host disease post bone marrow transplant (4%), celiac disease with sarcoidosis and chronic liver disease (4%), and tuberculosis (4%). All the biopsies were reviewed and none of the biopsies had significant chronic changes. Only few patients showed chronic changes at the time of biopsy, that is, minimal (0–5%) interstitial fibrosis/tubular atrophy with <5% glomerulosclerosis and no evidence of arteriosclerosis/arteriolar hyalinosis. Children with PLA2R-negative iMN had higher degree of proteinuria (18.4 vs. 15.6, P = 0.84) and higher incidence of HTN (43% vs. 14, P = 0.00).
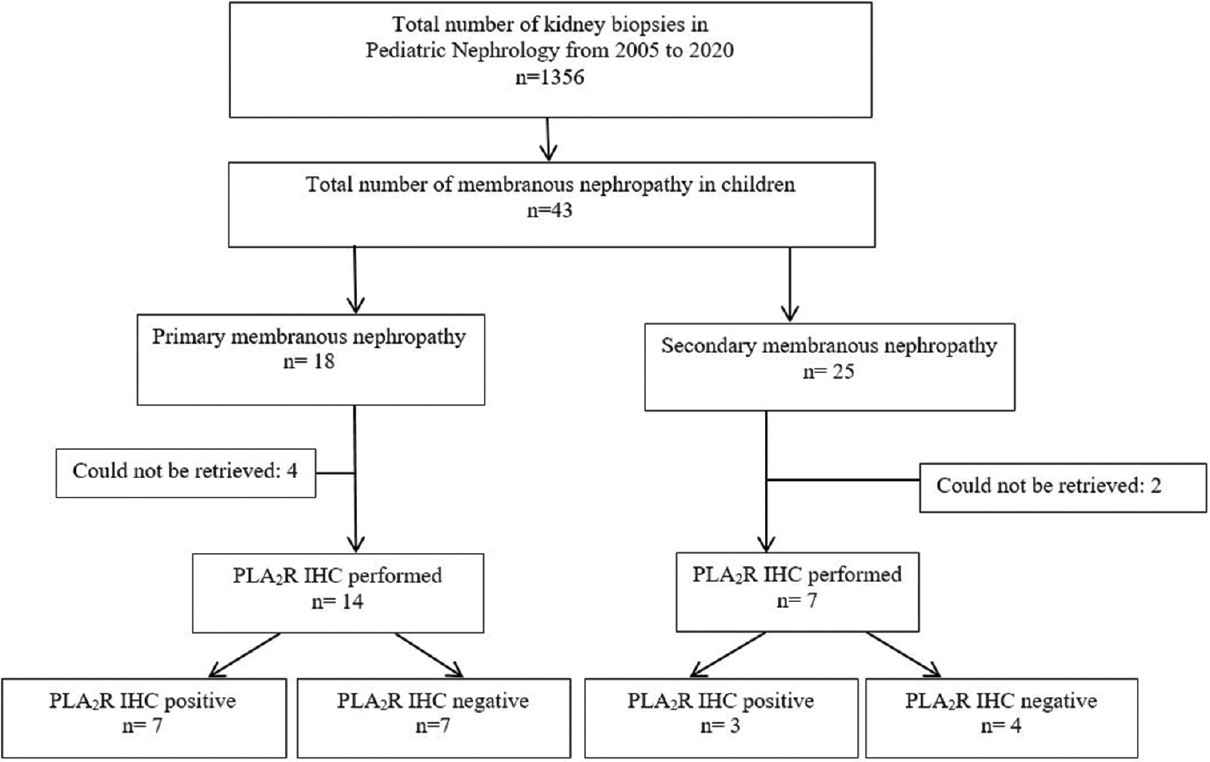
- Flow diagram of the study patients showing enrolment and follow up of 43 patients from 2005 to 2020
| Total MN (n=43) | iMN (n=18) | SMN (n=25) | P | |
|---|---|---|---|---|
| Age at onset (years) | 12 (10-14) | 12 (10.8-14) | 12 (9.5-14) | 0.44 |
| Gender (male:female ratio) | 0.8:1 | 1:1 | 0.6:1 | 0.55 |
| Age groups | ||||
| 1-5 years | 3 (7) | 1 (33) | 2 (67) | 0.04 |
| 6-10 years | 10 (23.3) | 3 (30) | 7 (70) | 0.04 |
| 11-15 years | 30 (69.7) | 14 (47) | 16 (53) | 0.97 |
| Follow-up (months) | ||||
| Median (range) | 22 (140) | 16 (140) | 30 (98) | 0.8 |
| uPCR | 6.9 (3.4-11.2) | 7 (3.7-25) | 6.9 (5.9-12) | 0.03 |
| Serum albumin, g/dl | 2.45 (1.99-2.8) | 2.25 (1.7-2.45) | 2.45 (1.99-2.8) | 0.50 |
| Nephrotic syndrome, n (%) | 23 (54) | 9 (50) | 14 (56) | 0.76 |
| eGFR (ml/min/1.73 m2) | 131 (111, 179) | 120 (105, 166) | 126 (82,143) | 0.94 |
| Microscopic hematuria, n (%) | 41 (95.3) | 17 (94.4) | 24 (96.0) | 0.53 |
| Hypertension, n (%) | 11 (25.6) | 5 (28.0) | 6 (24.0) | 0.53 |
eGFR=estimated glomerular filtration rate, iMN=idiopathic MN, IQR=interquartile range, MN=membranous nephropathy, SMN=secondary MN, uPCR=urine protein creatinine ratio. Categorical variables are represented as n (%) and continuous variables as median (IQR). Significant P values have been highlighted as bold
Treatment received
40% (n = 7/18) of iMN and 28% (n = 7/25) of SMN children received only corticosteroids. Mycophenolate mofetil was added in one-third (n = 6/18, 33.3%) of iMN and more than half (n = 14/25, 56%) of SMN. Third most common immunosuppressive agent used was tacrolimus (12%). The details of treatment received have been summarized in Table 2. There was no significant difference in the clinical outcomes between the different immunosuppressive agents.
| Treatment received | Total (n=43) | iMN (n=18) | SMN (n=25) | P |
|---|---|---|---|---|
| RAAS blockade, n (%) | 41 (95.3) | 16 (89) | 25 (100) | 0.17 |
| Number of antihypertensives (mean±SD) | 1.33±0.92 | 1.44±1.2 | 1.2±0.7 | 0.48 |
| Previous immunosuppression (%) | 26 (60.5) | 10 (55.6) | 16 (64) | 0.40 |
| Need for second line, n (%) | 28 (11.4) | 11 (61.1) | 17 (68) | 0.44 |
| Treatment used | ||||
| No immunosuppression, n (%) | 1 (4) | 0 (0) | 1 (4) | 0.36 |
| Corticosteroid alone, n (%) | 14 (32.6) | 7 (38.9) | 7 (28) | |
| Corticosteroid + CYP, n (%) | 4 (9.3) | 3 (16.7) | 1 (4) | |
| Corticosteroid + Tac, n (%) | 5 (11.6) | 3 (16.7) | 2 (8) | |
| Corticosteroid + MMF, n (%) | 20 (46.5) | 6 (33.3) | 14 (56) | |
| Duration of Tac (months), median (range) | 18 (6-35) | 14 (6-19) | 27 (18-35) | 0.19 |
| Duration of MMF (months), median (range) | 27 (6-71) | 33 (9-71) | 25 (6-61) | 0.48 |
| Rituximab, n (%) | 2 (4.7) | 2 (11.1) | 0 (0) | 0.02 |
| Sequential use of multiple IMS (%) | 2 (4.7) | 2 (11.1) | 0 (0) | 0.02 |
CYP=cyclophosphamide, iMN=idiopathic MN, IMS=immunosuppression, MMF=mycophenolate mofetil, MN=membranous nephropathy, RAAS=renin-angiotensin-aldosterone system, SD=standard deviation, SMN=secondary MN, Tac=tacrolimus
Response to therapy and outcome
Mean time to CR in iMN and SMN was 14 (4.5, 34.5) and 8.5 (3.8, 14.3) months, respectively (P = 0.13). The proportion of patients with CR/PR at the last follow-up was 86% (n = 12/14) in iMN and 90.9% (n = 20/22) in SMN (P = 0.59). Figure 3a shows the Kaplan–Meier graph depicting the response rate among the patients with iMN and SMN, showing that SMN had better remission rates and iMN took longer time for remission, though the difference was not statistically significant. Number of patients who had persistent proteinuria was significantly more in iMN (55.5% vs. 23%) in the first year and at the final visit (93% vs. 4.6%) (P = 0.01). iMN had more relapses (0.44 ± 0.98) than SMN (0.24 ± 0.58) (P = 0.38). CKD 5 developed in 2/14 (14.3%) iMN patients and both were PLA2R positive. The long-term follow-up and outcome of children with MN is presented in Table 3. Response to therapy assessed as CR or PR at various intervals is depicted in Figure 4. Children with PLA2R-negative iMN had higher degree of proteinuria (18.4 vs. 15.6, P = 0.84) and higher incidence of HTN (43% vs. 14%, P = 0.00). The time taken to achieve PR was more in PLA2R-negative children (5.7 ± 6.3 months) than PLA2R-positive children (2 ± 3.3 months), and none of the PLA2R-negative iMN had attained CR. PLA2R-negative children had higher persistent proteinuria at the end of therapy. Figure 3b depicts the response rate among the patients with PLA2R-positive and -negative iMN. Though PLA2R-negative iMN children were noted to have more severe clinical presentation at the onset and took longer time to respond to treatment, there were no statistically significant differences between the two groups on long-term follow-up in terms of remission. The details of PLA2R-positive and -negative subgroups are given in Supplementary Tables 1–3.
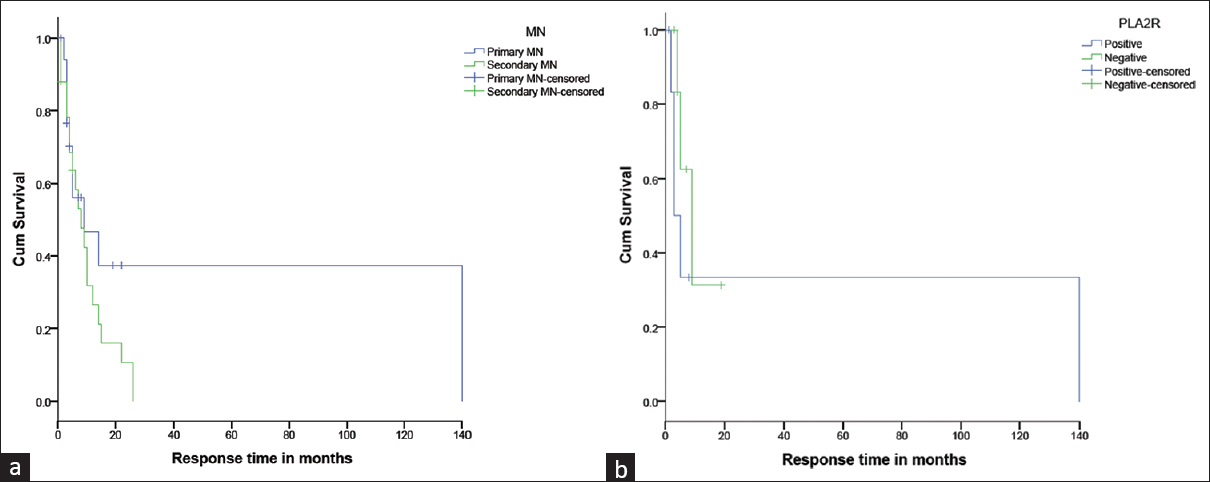
- (a) Mean time to complete remission in iMN and SMN were 14 (4.5, 34.5) and 8.5 (3.8, 14.3) months respectively (p=0.13). The proportion of patients with CR/PR at last follow up was 86% (n=12/14) in iMN and 90.9% (n=20/22) in SMN (p=0.59). (b) There were no statistically significant differences between the two groups who were PLA2r positive and negative on long term follow up in terms of partial or complete remission
| Total (n=43) | iMN (n=18) | SMN (n=25) | P | |
|---|---|---|---|---|
| Time to CR | 9 (4-15) | 14 (4.5-34.5) | 8.5 (3.8-14.3) | 0.13 |
| Presence of relapses | 9 (20.9) | 4 (22) | 5 (20) | 0.58 |
| Number of relapses, ean±SD | 0.33±0.75 | 0.44±0.98 | 0.24±0.5 | 0.38 |
| Patients with persistent proteinuria at 1 year | 8/22 (36.4) | 5/9 (55.5) | 3/13 (23) | 0.01 |
| Patients with persistent proteinuria at the final visit | 14/36 (38.9) | 13/14 (93) | 1/22 (4.6) | 0.00 |
| Mean uPCR at 1 year | 1.54±3.18 | 2.67±4.5 | 0.76±1.7 | 0.17 |
| Mean uPCR at the final visit | 1.7±3.5 | 2.3±3.7 | 1.33±3.4 | 0.39 |
| 3 months follow-up, n (%) | n=31 (72.1) | n=14 (77.8) | n=17 (68) | |
| CR | 3 (9.7) | 1 (7.1) | 2 (11.8) | 0.86 |
| Partial remission | 18 (58.1) | 8 (57.1) | 10 (58.8) | |
| Nonresponse | 10 (32.3) | 5 (35.7) | 5 (29.4) | |
| Mean eGFR | 142 (108-162) | 134 (108-173) | 119 (78-148) | 0.94 |
| 6 months follow-up, n (%) | n=30 (69.8) | n=12 (66.7) | n=18 (72) | |
| CR | 9 (30) | 1 (8.3) | 8 (44.4) | 0.74 |
| Partial remission | 18 (60) | 9 (75) | 9 (50) | |
| Nonresponse | 3 (10) | 2 (16.7) | 1 (5.6) | |
| Mean eGFR | 126 (105-158) | 115 (97-137) | 118 (98-151) | 0.74 |
| 12 months follow-up, n (%) | n=22 (51.2) | n=9 (50) | n=13 (52) | |
| CR | 11 (50) | 2 (22.2) | 9 (69.2) | 0.13 |
| Partial remission | 8 (36.4) | 5 (55.6) | 3 (23) | |
| Nonresponse | 3 (13.6) | 2 (22.2) | 1 (7.7) | |
| Mean eGFR | 132 (86-156) | 130 (54-156) | 131 (85-150) | 0.14 |
| 24 months follow-up, n (%) | n=17 (39.5) | n=6 (33.3) | n=11 (44) | |
| CR | 8 (47.1) | 0 | 8 (72.7) | 0.03 |
| Partial remission | 8 (47.1) | 5 (83.3) | 3 (27.3) | |
| Nonresponse | 1 (5.9) | 1 (16.7) | 0 | |
| Mean eGFR | 118 (97-142) | 136 (115-207) | 118 (98-135) | 0.12 |
| Final follow-up, n (%) | 36 (83.7) | 14 (77.8) | 22 (88) | |
| CR | 18 (50) | 1 (7.1) | 17 (77.3) | 0.00 |
| Partial remission | 14 (38.9) | 11 (78.6) | 3 (13.63) | |
| Nonresponse | 4 (11.1) | 2 (14.3) | 2 (9.1) | |
| Mean eGFR | 129 (95-152) | 108 (39-135) | 136 (105-149) | 0.06 |
| CKD 5D at final visit, n (%) | 2 (5.6) | 2 (14.3) | 0 | 0.17 |
CKD 5D=chronic kidney disease stage 5, CR=complete remission, eGFR=estimated glomerular filtration rate, iMN=idiopathic membranous nephropathy, IQR=interquartile range, SMN=secondary membranous nephropathy, uPCR=urine protein creatinine ratio. Categorical variables are represented as n (%) and continuous variables as median (IQR). Significant P values have been highlighted as bold

- Response rates were measured by uPCR at various intervals of follow up and values expressed in percentages. CR: complete remission, PR: partial remission, NR: non-remission, eGFR estimated GFR
| Outcome parameters | iMN | SMN | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower CI | Upper CI | P | OR | Lower CI | Upper CI | P | |
| Age above 10 years | 1.79 | 0.39 | 8.25 | 0.46 | 0.91 | 0.34 | 2.42 | 0.85 |
| Female gender | 1.37 | 0.43 | 4.30 | 0.59 | 1.39 | 0.52 | 3.70 | 0.51 |
| Hypertension | 0.29 | 0.06 | 1.36 | 0.12 | 3.50 | 0.98 | 12.54 | 0.05 |
| Nephrotic proteinuria at the onset | 0.84 | 0.17 | 4.18 | 0.83 | 0.33 | 0.04 | 2.64 | 0.30 |
| PLA2R positive | 0.76 | 0.21 | 2.73 | 0.67 | 2.83 | 0.17 | 47.15 | 0.47 |
| Presence of relapse | 0.30 | 0.06 | 1.43 | 0.13 | 0.74 | 0.26 | 2.12 | 0.57 |
| Treatment with tacrolimus | 3.22 | 0.33 | 31.49 | 0.31 | 0.73 | 0.09 | 5.66 | 0.77 |
| Treatment with CYP | 0.27 | 0.03 | 2.12 | 0.21 | 0.73 | 0.09 | 5.66 | 0.77 |
| Treatment with MMF | 1.15 | 0.38 | 3.50 | 0.80 | 1.06 | 0.40 | 2.80 | 0.91 |
CI=confidence interval, CYP=cyclophosphamide, iMN=idiopathic membranous nephropathy, MMF=mycophenolate mofetil, OR=odds ratio, PLA2R=anti-phospholipid A2 receptor antibody, SMN=secondary membranous nephropathy
| Total iMN with PLA2R IHC (n=14) | PLA2R pos (n=7) | PLA2R neg (n=7) | P | |
|---|---|---|---|---|
| Age: Mean±SD | 13 (10.8, 14.3) | 14 (8.4, 16) | 12 (10,14) | 0.88 |
| Gender M: F | 0.8:1 | 1.3:1 | 0.75:1 | 0.00 |
| Age groups | ||||
| 1-5 years | 1 (7.1) | 1 (14.3) | 0 (0) | 0.032 |
| 6-10 years | 2 (14.3) | 0 (0) | 2 (28.6) | 0.04 |
| 11-15 years | 11 (78.6) | 6 (85.7) | 5 (71.4) | 0.84 |
| uPCR | 7.6 (4.9, 15.2) | 7.29 (3,40) | 8.9 (7, 38) | 0.84 |
| Serum albumin g/dL | 1.9 (1.3, 3.4) | 1.9 (1.3,2.7) | 2.3 (1.3,3.4) | 0.33 |
| eGFR (ml/min/1.73m2) mean±SD | 156±81 | 184±116 | 154±43 | 0.54 |
| Microscopic hematuria, n (%) | 13 (92.9) | 7 (100) | 6 (85.7) | 0.00 |
| Hypertension, n (%) | 4 (28.6) | 1 (14.3) | 3 (42.9) | 0.00 |
Categorical variables are represented as n (%) and continuous variables as median (IQR). eGFR estimated glomerular filtration rate, iMN idiopathic MN, PLA2R anti phospholipase A2 receptor antibody uPCR urine protein creatinine ratio
| iMN with PLA2R IHC (n=14) | PLA2R Pos (n=7) | PLA2R Neg (n=7) | P | |
|---|---|---|---|---|
| RAAS blockade | 14 (100) | 7 (100%) | 7 (100%) | 0.60 |
| Number of anti HTN drugs (Mean±SD) | 1.71±1.1 | 1.29±0.76 | 2.14±1.4 | 0.17 |
| Prior immunosuppressive treatment | 7 (50) | 3 (42.9) | 4 (57.1) | 0.5 |
| Need for second line | 11 (78.6) | 5 (71.4) | 6 (85.7) | 0.00 |
| No immunosuppression n (%) | 0 (0) | 0 (0) | 0 (0) | 0.00 |
| Corticosteroid alone n (%) | 3 (21.4) | 2 (28.6) | 1 (14.3) | |
| Corticosteroid + CYP n (%) | 2 (14.3) | 1 (14.3) | 1 (14.3) | |
| Corticosteroid + Tac n (%) | 2 (14.3) | 1 (14.3) | 1 (14.3) | |
| Corticosteroid + MMF n (%) | 5 (35.7) | 1 (14.3) | 4 (57.1) | |
| Rituximab n (%) | 2 (14.3) | 2 (28.6) | 0 (0) | |
| Sequential use of multiple IMS n (%) | 2 (14.3) | 1 (14.3) | 1 (14.3) |
RAAS: Renin angiotensin aldosterone system, HTN: Hypertension, MMF: Mycophenolate mofetil, Tac: Tacrolimus, CYP: Cyclophosphamide, IMS: Immunosuppression
| iMN with PLA2R IHC (n=14) | PLA2R Pos (n=7) | PLA2R Neg (n=7) | P | |
|---|---|---|---|---|
| Presence of relapses | 4 (28.6) | 2 (28.6) | 2 (28.6) | 0.72 |
| Number of relapses, Mean | 0.57±1.1 | 0.57±1.1 | 0.57±1.1 | 1.00 |
| Proportion of patients with persistent proteinuria at 1 year | 5 (35.7) | 3 (42.9) | 2 (28.6) | 0.00 |
| Proportion of patients with persistent proteinuria at final visit | 12 (85.7) | 6 (85.7) | 6 (85.7) | 1.00 |
| 3 months follow up | n=13 (92.9) | n=6 (85.7) | n=7 (100) | 0.00 |
| Complete remission | 0 | 0 | 0 | |
| Partial remission | 3 (50) | 4 (66.7) | 4 (57.1) | |
| Non -response | 3 (50) | 2 (33.3) | 3 (42.9) | |
| Mean eGFR (Mean±SD) | 139±41.8 | 123±49 | 151±32 | 0.24 |
| 6 months follow up | n=10 (71.4) | n=5 (71.4) | n=5 (71.4) | 0.00 |
| Complete remission | 1 (10) | 0 | 1 (20) | |
| Partial remission | 6 (60) | 3 (60) | 3 (60) | |
| Non -response | 3 (30) | 2 (40) | 1 (20) | |
| Mean eGFR (Mean±SD) | 133±30 | 134±42 | 132±17 | 0.95 |
| 12 months follow up | n=11 (78.6) | n=6 (85.7) | n=5 (71.4) | |
| Complete remission | 1 (9.1) | 0 | 1 (20) | 0.00 |
| Partial remission | 8 (72.7) | 5 (83.3) | 3 (60) | |
| Non -response | 2 (18.2) | 1 (16.7) | 1 (20) | |
| Mean eGFR (Mean±SD) | 98±60 | 90.66±51 | 137±31.60 | 0.25 |
| 24 months follow up | n=5 (35.7) | n=3 (42.9) | n=2 (28.6) | 0.14 |
| Complete remission | 0 | 0 | 0 | |
| Partial remission | 4 (80) | 1 (33.3) | 2 (100) | |
| Non -response | 1 (20) | 2 (66.7) | 0 | |
| Mean eGFR (Mean±SD) | 156±49 | 155±52 | 168±46 | 0.79 |
| Final follow up | n=13 (92.9) | n=6 (85.7) | n=7 (100) | 0.00 |
| Complete remission | 0 | 0 | 0 | |
| Partial remission | 10 (76.9) | 3 (50) | 6 (83.3) | |
| Non -response | 3 (23) | 3 (50) | 1 (14.3) | |
| Mean eGFR (Mean±SD) | 106±54 | 89±68 | 123±34 | 0.31 |
| CKD 5D at final follow up | 2 (14.3) | 2 (28.6) | 0 | 0.00 |
Categorical variables are represented as n (%) and continuous variables as median (IQR)
As shown in Table 4, we performed univariate logistic regression with outcome parameters for assessing the risk factors for nonresponse at 6 months. The regression analysis did not show any significant association with outcome.
Discussion
IHC PLA2R staining of glomerular tissue is a useful diagnostic marker of iMN. Tissue PLA2R had a poor diagnostic accuracy (52%; 95% confidence interval [CI]:30%, 74%) for diagnosing iMN in children. The outcome with respect to CR or PR was not significantly different in iMN and SMN [Figure 4]. PLA2R-negative iMN children were noted to have more severe clinical presentation at the onset and took longer time to respond to treatment in terms of remission.
Response rate of iMN was 83%, 78%, 83%, and 86% at 6 months, 1 year, 2 years, and final follow-up in our study. Clinical response rate reported by Lee et al.[19] was 65% and by Valentini et al.[18] was 75%. In a study among adolescent MN patients by Kumar et al.,[14] clinical remission at the end of 6 and 12 months of therapy was seen in 61% and 50% of patients, respectively. Response rate was found to be better in SMN, that is, 94%, 92%, 100%, and 91% at 6 months, 1 year, 2 years, and final follow-up. At the last follow-up, Menon et al.[28] showed 86% and 95% children with iMN and SMN, respectively, to have a good response to therapy (CR/PR). Moroni et al[29] reported similar remission rate of 90% at 83 months in lupus MN.
The response in our series was similar irrespective of the choice of immunosuppressive agents. Mycophenolate mofetil was the common second-line agent (46%). Corticosteroid monotherapy, which is not obsolete in children, was used in a subset of patients (33%).
Rituximab was used in our study in two non-responders. Efficacy of rituximab in pediatric iMN was 75% in the Indian cohort studied by Ramachandran et al.[30] Two regimens are being commonly practiced: four doses of 375 mg/m2 weekly apart or two doses of 375 mg/m2 at an interval of 2 weeks.[31-34]
Chen et al.[35] noted that one-fourth of patients progressed to CKD 3 at a mean follow-up of 42 months. Risk factors for poor prognosis reported in children with iMN are older age, presence of NS or HTN, low glomerular filtration rate (GFR) at presentation, kidney biopsy stage 3 and 4, presence of segmental sclerosis, and tubulointerstitial damage on kidney biopsy and response to treatment.[36-39] Due to rarity of the condition, ascertaining the optimum immunosuppressive regimen for childhood iMN is difficult. The clinical course of PLA2R-positive patients was protracted when compared to PLA2R-negative patients.
Limitations
Though we have described a significant number of children (n = 43) with MN followed from a single center in India, it is truly difficult to conclude and generalize these findings with the present numbers. PLA2R staining could not be done in all the kidney biopsy specimens of iMN as paraffin blocks could not be retrieved from the archives. Serum anti-PLA2R evaluation was possible only in some patients, and hence, their data was not summarized. In view of the small number of patients in each subgroup, multivariate regression analysis could not be performed.
Conclusion
Individualized and targeted immunosuppression will help to improve response to therapy and long-term outcome. Larger studies are needed in children to ascertain the implications of PLA2R in childhood iMN. The latest guidelines for management in adult MN are guided by serum PLA2R level. Hence, regular testing could help monitor disease activity in children also. Newer antigens which could have variable response to immunosuppressive therapy need further research in children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The pathology technicians are acknowledged for retrieving the kidney biopsy specimens from the pathology archives.
References
- Clinical and histological features of phospholipase A2 receptor-associated and thrombospondin type-I domain-containing 7A-associated idiopathic membranous nephropathy:A single center retrospective study from China. Med Sci Monit. 2018;24:5076-83.
- [Google Scholar]
- Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19:653-60.
- [Google Scholar]
- M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11-21.
- [Google Scholar]
- Compared staining of the phospholipase A2 receptor in the glomeruli of Chinese adults and children with idiopathic membranous nephropathy. Pathol Res Pract. 2019;215:952-6.
- [Google Scholar]
- Phospholipase A2 receptor staining in pediatric idiopathic membranous glomerulopathy. Pediatr Nephrol. 2013;28:2307-11.
- [Google Scholar]
- Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277-87.
- [Google Scholar]
- Antigens in membranous nephropathy:Progress toward precision. Am J Kidney Dis. 2020;76:610-12.
- [Google Scholar]
- Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020;98:1253-64.
- [Google Scholar]
- Membranous nephropathy:A single disease or a pattern of injury resulting from different diseases. Clin Kidney J. 2021;14:2166-9.
- [Google Scholar]
- An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526-32.
- [Google Scholar]
- PLA2R in Glomerular deposit and anti PLA2R antibodies in Indian patients with active IMN. Nephrology. 2014;19((Suppl 2)):23-76.
- [Google Scholar]
- Antibodies to m-type phospholipase A2 receptor in children with idiopathic membranous nephropathy. Nephrology. 2015;20:572-5.
- [Google Scholar]
- Immunohistochemical glomerular expression of phospholipase A2 receptor in primary and secondary membranous nephropathy:A retrospective study in an Indian cohort with clinicopathological correlations. Nephron Extra. 2017;7:1-9.
- [Google Scholar]
- Prevalence of serum anti M-type phospholipase A2 receptor antibody in primary membranous nephropathy:A single center experience. Indian J Nephrol. 2016;26:257-61.
- [Google Scholar]
- Retrospective study of phospholipase A2 receptor and IgG subclasses in glomerular deposits in chinese patients with membranous nephropathy. PLoS One. 2016;11:e0156263.
- [Google Scholar]
- Membranous glomerulonephritis:Treatment response and outcome in children. Pediatr Nephrol. 2009;24:301-8.
- [Google Scholar]
- A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421-30.
- [Google Scholar]
- KDIGO clinical practice guideline for glomerulonephritis. Kidney Int (Suppl 2):139-274.
- [Google Scholar]
- Management and treatment of glomerular diseases (part 1):Conclusions from a Kidney Disease:Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:268-80.
- [Google Scholar]
- The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114((2 Suppl)):555-76.
- [Google Scholar]
- The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571-90.
- [Google Scholar]
- Steroid sensitive nephrotic syndrome:Revised guidelines. Indian Pediatr. 2021;58:461-81.
- [Google Scholar]
- Membranous nephropathy:A journey from bench to bedside. Am J Kidney Dis. 2016;68:138-47.
- [Google Scholar]
- Membranous nephropathy in children:Clinical presentation and therapeutic approach. Pediatr Nephrol. 2010;25:1419-28.
- [Google Scholar]
- Primary membranous nephropathy in children and adolescents:A single-centre report from South Asia. Pediatr Nephrol. 2021;36:1217-26.
- [Google Scholar]
- Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36-46.
- [Google Scholar]
- Successful treatment and clearing of circulating CD19-positive cells by rituximab in a child with idiopathic membranous nephropathy. Pediatr Nephrol. 2011;26:637-8.
- [Google Scholar]
- Idiopathic membranous nephropathy in children treated with rituximab:Report of two cases. Pediatr Nephrol. 2018;33:1089-92.
- [Google Scholar]
- Idiopathic membranous nephropathy in pediatric patients:Presentation, response to therapy, and long-term outcome. BMC Nephrol. 2007;8:11.
- [Google Scholar]
- Idiopathic membranous glomerulopathy in Canadian children:A clinicopathologic study. J Pediatr. 1982;101:682-5.
- [Google Scholar]
- Prognostic factors in idiopathic membranous nephropathy. Am J Kidney Dis. 1998;31:1-11.
- [Google Scholar]







