Translate this page into:
Clinical Profile and Predictors Affecting Outcome in Community-Acquired Acute Kidney Injury: A 3 Months Follow-Up Study
Corresponding author: Muthukumar Balakrishnan, Department of Nephrology, Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr Ram Manohar Lohia (Dr RML) Hospital, New Delhi, India Email: itzmedrmuthu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tarachandani R, Pursnani L, Balakrishnan M, Mahapatra HS, Bhattacharyya S, Chaudhary P, et al. Clinical Profile and Predictors Affecting Outcome in Community-Acquired Acute Kidney Injury: A 3 Months Follow-Up Study. Indian J Nephrol. 2024;34:475-81. doi: 10.25259/ijn_352_23
Abstract
Background
Community-Acquired Acute Kidney Injury (CA-AKI) is often a devastating clinical syndrome allied with high hospital mortality. Moreover, only limited prospective data exist on the outcomes of CA-AKI. Hence, this follow-up study was conducted to assess clinical profiles and the factors affecting outcomes in CA-AKI.
Materials and Methods
A prospective study enrolling 283 participants was conducted from the year 2021 to 2022. AKI patients defined as per Kidney Disease Improving Global Outcomes (KDIGO) criteria were included. Data were collected on demographics, clinical features, and etiological factors. Patients were followed for three months. Univariate and multinomial analyses were done to predict outcomes. The Cox regression model was fitted to identify predictors of mortality.
Results
The mean age of patients was 41.67±16.21 years with male predominance. Most of the patients required non-ICU (81.9%) care. Around 36% and 39.6 % of AKI patients were oliguric and required dialysis, respectively. Most patients had a single etiology, with sepsis being the most common cause. Most patients were in KDIGO stage 3, followed by stage 2. At three months of follow-up, 40.6%, 12.3%, and 4.2% had complete, partial, and non-recovery, respectively, and 30.4% died. Age, single etiology, hepatorenal syndrome, sepsis, requirement of mechanical ventilation and vasopressors, comorbidities and glomerulonephritis were significantly associated with mortality.
Conclusion
CA-AKI is significantly associated with higher mortality, even for those patients who require non-ICU care on presentation. This highlights the pressing need for AKI prevention, early detection, and intervention to mitigate reversible risk factors and optimize clinical outcomes.
Keywords
Community-acquired acute kidney injury
AKI
Sepsis
KDIGO
Recovery pattern
Introduction
Acute kidney injury (AKI) is one of the major complications in acutely ill patients and imposes significant mortality and morbidity globally.1 AKI may be present during admission to the hospital or develop during hospitalization.1 In tropical countries like India, community-acquired AKI (CA-AKI), which occurs outside the hospital setting, is due to dehydration, diarrhea, infections, venomous snakebite, etc.2 Within the Indian subcontinent, there is a significant variation in the etiology of CA-AKI reported across distant geographical areas, and the etiologic spectrum has been demonstrated to change over time, ranging from malaria, sepsis, nephrotoxic drugs, liver disease3 to diarrhea, glomerulonephritis, sepsis, snakebite, and leptospirosis.2
CA-AKI is a common and often devastating clinical syndrome allied with a high in-hospital mortality rates.4 Among survivors, severe CA-AKI requiring dialysis can result in non-recovery or incomplete progressing to chronic kidney disease (CKD).5 Patients who develop AKI are at considerable risk for the progression of CKD by 1 year following hospitalization, even for the less severe forms of AKI.6 Recently, there has been rising recognition that even AKI patients with apparent complete recovery remain at risk for progression to CKD.7
There are several gaps in our knowledge regarding the pathophysiology and clinical course of AKI, which does not recover.8 There is a need to consider competing risk factors when assessing recovery patterns.9 However, most studies with available clinical data have had limitations, including being comparatively small and regional, incapable of accounting for crucial confounding factors such as proteinuria, etc.7 Such studies also do not permit exhaustive characterization of renal outcome patterns.
Timing of renal function recovery after AKI is associated with an increased risk of long-term progression to CKD and also affects survival rates.4,10 There is a necessity to improve the outcomes of AKI partial and non-recovery; however, there is very scanty literature available on the follow-up of these AKI patients.8 Further, few studies are available from developing countries. Among those available, majority of them were conducted on hospital-acquired AKIs. Thus, this study was conducted to evaluate risk factors for developing CA-AKI, the pattern of renal recovery, and their 3-month outcomes.
Materials and Methods
This prospective observational study was conducted at the Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and its associated Dr. Ram Manohar Lohia Hospital (RMLH), a high-volume tertiary care hospital in New Delhi, India, after institutional ethics committee approval and patient consent was obtained. The study was conducted on patients with more than 18 years of age, and those satisfying AKI as per KDIGO criteria were included if AKI was present at presentation or developed within 48 h of hospital admission, labeling them as CA-AKI.2 Those patients with established CKD, probable CKD with a surrogate marker with imaging showing any structural abnormalities, or bilaterally reduced kidney size less than 8.5 cm or on any form of renal replacement therapy (RRT) were excluded from the study.
The recruitment of patients for the study involved receiving referrals for nephrology consultations from various departments. The inclusion criteria encompassed both out- and in-patient cases within the nephrology department, as well as individuals referred from other wards. Data abstraction checklists and structured proforma were developed. The information about the patient’s demographics, diagnosis, and associated comorbidities was collected, and laboratory investigation results (creatinine, urea, electrolytes, complete blood count, and liver function tests) were noted. All patients underwent an ultrasonogram of the kidney to note the size and the structural abnormalities. Other additional investigations were done as warranted by clinical presentation; in patients presenting with signs and symptoms of sepsis, samples of blood culture and different cultures as necessitated were collected. The patients with features of glomerular diseases (hypertension, proteinuria, hematuria) underwent additional immunological and other investigations required to manage those patients.
All participants were followed up and assessed for the outcomes on day 7, at 1 month and 3 months. The patients’ clinical examination findings were noted, including the quantification of urine output by 24-h urinary volume and laboratory parameters, such as serum creatinine. Urine analysis was performed to detect proteinuria and microscopic hematuria.
The following were considered outcome variables:
(i) Complete recovery (CR) – a patient whose serum creatinine decreased and reached their baseline values, if available. If baseline value was unavailable, serum creatinine decreasing to less than or equal to 1 mg/dl was considered CR. For CR, the choice of a serum creatinine cut-off of 1 mg/dL was rooted in the assumption that this level in adults maintains a glomerular filtration rate (GFR) above 60 ml/min across various age groups. This decision was made considering the pragmatic realities of our study setting and was further supported by consultation references where other departments might be unaware of GFR calculations.
(ii) Partial recovery (PR) – a patient who did not achieve baseline creatinine or whose serum creatinine was more than 1 mg/dl but decreased to less than 4 mg/dl (as per KDIGO stage 3, using a creatinine level of 4 mg/dl as the cut-off)11 and did not require dialysis.
(iii) Non-recovery (NR) – a patient whose serum creatinine was more than 4 mg/dl, not requiring dialysis or any patient requiring dialysis support.
(iv) Death of the patient.
Baseline creatinine was the value of creatinine in mg/dl available from 8 days to 12 months before the current presentation with CA-AKI. In the absence of studies demonstrating the validity of serum creatinine or GFR imputation in the Indian population, an empirical cut-off value of serum creatinine was used. In defining NR, serum creatinine of more than 4 mg/dl was chosen, as this creatinine value was considered a severe form of AKI by KDIGO. Imputing this creatinine value for any adult placed them into advanced renal dysfunction. At each follow-up, the patients were assessed for outcome status. The study endpoints were the achievement of CR or the death of the patient.
As per the study by Korula et al.,12 the minimum sample required was calculated using the formula: N ≥ Z2(p(100-p))/d2, considering the p (prevalence of disease) as 16.1% and margin of error (d) as 5%, at 95% confidence level, the calculated sample size was 217. Considering the dropout rate of 10% at each follow-up at 7 days, 1 month, and 3 months, respectively, with a total dropout rate of 30%, the minimum sample size required was 283 participants.
The data entry was done in Microsoft Excel, and analysis was done using Epi info software 7.2.2.2. The normality distribution of the data was tested by using the Kolmogorov–Smirnov test. Baseline characteristics and patterns of AKI were presented as numbers and percentages. The quantitative data were presented as means ± SD. A bar diagram was used to display the pattern of AKI outcomes at each follow-up period. Univariable Cox regression was carried out to evaluate the effects of potential factors on overall survival. The multivariable analysis included all the variables with a p-value of <0.05 by univariable Cox regression. Reduced model selection using Akaike’s information criterion was done to build a multivariate model. Multinomial logistic regression was used to calculate the adjusted odds ratio (AOR) following univariate analysis of overall patient outcome. For all analyses, the p-value is taken as statistically significant when it is less than 0.05.
Results
Table 1 displays the baseline and clinical characteristics of study participants. The mean age of participants was 41.67 ± 16.21 years. Most participants, with male predominance, fell into the 18–45 age range. Comorbidities were observed in approximately 27% of patients, with hypertension and diabetes being the common conditions. Most participants required non-ICU care and were admitted to the nephrology or medicine ward. Baseline creatinine was available in only 11 patients.
| Variables | Number, n (%)/Mean ± SD |
|---|---|
| Age (years) | 41.67 ± 16.21 |
| 18–45 years | 172 (60.7) |
| 46–60 years | 74 (26.1) |
| ≥61 years | 37 (13.1) |
| Male gender | 159 (56.2) |
| Associated comorbidities | 79 (27.9) |
| Diabetes mellitus | 34 (12) |
| Hypertension | 44 (15.5) |
| Cardiovascular disease | 9 (3.2) |
| Cerebrovascular accident | 5 (1.8) |
| Place of admission | |
| Non-ICU | 232 (81.9) |
| ICU | 51 (18.1) |
| Causes | |
| Medical | 218 (77.1) |
| Surgical | 60 (21.2) |
| Obstetric | 5 (1.7) |
| Type of AKI | |
| Oliguric | 103 (36.0) |
| Non-oliguric | 180 (64.0) |
| KDIGO stage | |
| Stage 1 | 41 (14.5) |
| Stage 2 | 83 (29.3) |
| Stage 3 | 159 (56.2) |
| AKI etiology | |
| Single | 183 (64.7) |
| Multiple | 100 (35.3) |
| Patient on mechanical ventilatory support | 44 (15.5) |
| RRT requirement | 112 (39.6) |
SD - Standard deviation, AKI - Acute kidney injury, ICU - Intensive care unit, KDIGO - Kidney Disease: Improving Global Outcomes, RRT - Renal replacement therapy. Multifactorial - Multiple coexisting etiologies considered. Categorical variables have been displayed in frequency and percentages. Continuous variables have been displayed as mean and standard deviation.
The majority of participants had oliguric AKI. As defined by KDIGO, stage 3 was commonly observed at AKI diagnosis, followed by stage 2 and stage 1 respectively. Most patients had a single etiology of AKI, with sepsis being the most common, followed by hypovolemia and glomerulonephritis. Mechanical ventilatory (MV) support and RRT were required in 15.5% and 39.6% of patients, respectively. Table 2 displays various possible causes of AKI among study participants.
| Causes of AKI | Frequency, n (%) |
|---|---|
| Pre-renal | |
|
Volume loss Gastrointestinal Third space Skin Renal |
61(21.6) 39 (13.8) 13 (4.6) 6 (2.2) 3 (1.1) |
|
Evidence of shock Septic shock Hypovolemic shock Cardiogenic shock |
54 (19.1) 32 (11.3) 12 (4.2) 10 (3.6) |
| Cardiorenal syndrome | 17 (6) |
| Hepatorenal syndrome | 9 (3.2) |
| Renal | |
| Sepsis-associated | 158 (55.8) |
| Glomerulonephritis | 52 (18.4) |
| Drug-induced | 28 (9.9) |
| Rhabdomyolysis | 13 (4.6) |
| Post-renal | |
| Calculus | 9 (3.2) |
| Non-calculi | 3 (1.1) |
AKI: Acute kidney injury. Categorical variables have been displayed in frequency and percentages. Pre-renal, Renal, and post-renal causes are multiple-choice variables.
In this study involving 283 patients, a significant prevalence of sepsis was identified, with 158 cases having discernible initial foci. The gastrointestinal tract emerged as the most prevalent site (30.4%), followed by the urinary tract (17.1%), and sepsis with an unspecified organ focus (16.5%). The respiratory tract and skin contributed 13.9% and 13.3%, respectively. The female genital tract, musculoskeletal system, and infective endocarditis comprised 5.7%, 2.5%, and 0.6%, respectively [Table S1].
The distribution of nephrotoxic drugs among the studied population (n = 28) revealed diverse contributors to renal impairment. Non-steroidal anti-inflammatory drugs (NSAIDs) were the most prevalent, accounting for 46.3% of cases, followed by alternative medications (17.9%), anti-tubercular drugs (7.1%), and others, each contributing to one case [Table S2].
Outcomes of AKI
Figure 1 displays the schematic presentation of AKI patient outcomes at various follow-ups. During the initial assessment on seventh day, it was observed that only 13.4% of patients had complete recovery, which increased to 40.6% by the third month follow-up. Partial recovery and non-recovery decreased with time by the final follow-up. At 3 months, 40.6%, 12.7%, and 4.2% of participants had complete, partial, and non-recovery, respectively. Mortality rates were 24.4%, 29.0%, and 30.4% at the follow-up of 7 days, 1 month, and 3 months, respectively. Excluding the GN patients, mortality rates were 28.1%, 32.5%, and 32.9% at the follow-up of 7 days, 1 month, and 3 months, respectively. Figure 2 illustrates the pattern of outcomes among study participants on day 7, 1 month, and 3 months of follow-up.

- Study flowchart. AKI: acute kidney injury; CKD: chronic kidney disease; FU: follow-up.
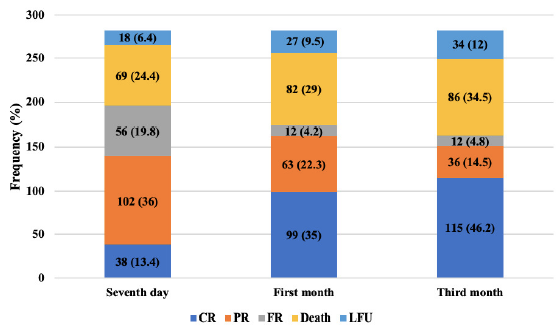
- Distribution of study participants (n = 283) based on the patient outcome on 7 days, 1 month and 3-month follow-up. CR: complete recovery; PR: partial recovery; FR: failure to recover; LFU: Lost to follow-up.
Predictors of mortality
Univariate Cox regression analysis identified factors associated with mortality among AKI patients. Age between 18 to 30 years (HR = 0.291, p < 0.001) and 31 to 45 years (HR = 0.534, p = 0.034), single etiology (HR = 0.482, p = 0.001), presence of glomerulonephritis (HR = 0.489, p = 0.034), higher serum protein (HR = 0.805, p = 0.01), and higher serum albumin (HR = 0.723, p = 0.007) were found to reduce the risk of mortality. Conversely, the presence of hepatorenal syndrome (HR = 3.801, p = <0.001), sepsis (HR = 2.606, p < 0.001), comorbidities (HR = 1.594, p = 0.035), and the requirement of mechanical ventilation (HR = 3.861, p < 0.001) and vasopressors (HR = 3.299, p < 0.001) were associated with increased probability of death [Table S3]. Subsequently, all statistically significant risk factors were subjected to multivariate Cox regression. The results in Table 3 show that the adjusted hazard ratio (AHR) was significant for only two factors: the presence of hepatorenal syndrome (AHR = 3.570, p = 0.006) and the requirement of ventilatory support (AHR = 2.406, p = 0.013).
| Variables | AHR | 95% CI | p-value |
|---|---|---|---|
| Age (years) | |||
| 18–30 | 0.48 | 0.221–1.043 | 0.064 |
| 31–45 | 0.825 | 0.425–1.602 | 0.57 |
| 46–60 | 0.849 | 0.457–1.578 | 0.604 |
| Single AKI etiology | 1.209 | 0.659–2.221 | 0.54 |
| Evidence of HRS | 3.57 | 1.453–8.769 | 0.006 |
| Cardiogenic shock | 0.758 | 0.14–4.108 | 0.748 |
| Hypovolemic shock | 0.72 | 0.191–2.714 | 0.627 |
| Septic shock | 0.684 | 0.244–1.912 | 0.469 |
| Evidence of GN | 0.82 | 0.373–1.804 | 0.622 |
| Evidence of sepsis | 1.988 | 0.989–3.993 | 0.054 |
| Patient on MV | 2.406 | 1.203–4.809 | 0.013 |
| Patient on vasopressor | 1.689 | 0.596–4.785 | 0.324 |
| Presence of comorbidities | 1.169 | 0.643–2.126 | 0.609 |
| Diabetes | 0.918 | 0.452–1.864 | 0.813 |
| Protein (gm/dl) | 0.965 | 0.742–1.256 | 0.793 |
| Albumin (gm/dl) | 0.887 | 0.637–1.236 | 0.48 |
AKI: acute kidney injury; HRS: hepatorenal syndrome; GN: glomerulonephritis; MV: mechanical ventilation; AHR: adjusted hazard ratio; CI: confidence interval. Reference group for age: ≥61 years, Reference group for AKI etiology: Multiple, Reference group for rest of the variables: Absence of the condition.
The data were intentionally analyzed, excluding the glomerulonephritis (GN) patients, as GN behaves differently from other causes, necessitating specific therapies. Even with this exclusion, the AHR remained significant for only two factors: the presence of hepatorenal syndrome (AHR = 3.425, p = 0.009) and the requirement for ventilatory support (AHR = 2.638, p = 0.008) [Table S4].
Factors predicting outcome measures (Complete recovery, Partial recovery, and Non-recovery)
Table S5 provides a comparison of various factors with patient outcomes. Factors such as age, etiology, hypovolemia, hepatorenal syndrome, shock, glomerulonephritis, sepsis, KDIGO stage, mechanical ventilatory, vasopressors, RRT requirement, comorbidities, obstetrics and gynecological disorders, serum creatinine, hemoglobin, total leucocyte count (TLC), and serum glutamic-oxaloacetic transaminase levels were found to have a significant association with outcome (p < 0.05). Subsequently, these factors were subjected to multinomial logistic regression [Table S6].
Partial recovery vs. Complete recovery: Among patients with partial recovery, factors such as age, glomerulonephritis, and RRT requirement at diagnosis significantly influenced outcomes compared to patients with complete recovery. For every 1-year increase in age, the probability of developing partial recovery increased by 1.056 times. The presence of glomerulonephritis and the requirement of RRT increased the chance of developing partial recovery by 4.8 and 5.2 times, respectively, compared to the complete recovery outcome [Table S6].
Non-recovery vs. Complete recovery: Among the patients with non-recovery, factors such as hemoglobin and glomerulonephritis significantly impacted patient outcomes compared to patients with complete recovery. An increase in hemoglobin was associated with a decreased probability of developing non-recovery. Conversely, the likelihood of non-recovery increased by 9.8 times in the presence of glomerulonephritis as an etiological factor [Table S6].
Discussion
The present study is one of a unique kind, addressing the risk factors of CA-AKI in a developing country and the outcome over 3 months of follow-up.
The present study found that patients were mainly among the younger age group, in accordance with the results of various studies.2,9,13,14 In contrast to our study findings, there are studies with the significant involvement of elderly patients.8,15–18 In developed countries, elderly patients tend to have a higher incidence of AKI, while younger populations are more commonly involved in developing countries.19 This difference may be because, in developing countries, AKI occurs mostly due to infectious and environmental causes. In contrast, developed countries experience a higher incidence of AKI in their elderly population, often due to complications arising from pre-existing medical conditions. Male predominance was observed in the current study, which is consistent with previous research.1,2,8,10 although Lee et al.16 reported 59.2% of females in their study.
Comorbidities were observed in 27.9% of participants, with hypertension and diabetes mellitus being common, in line with findings from various studies.1,2,11,13,20 The increased risk may occur due to pre-existing CKD, cardiovascular diseases, including acute coronary artery syndromes, hyperglycemic crisis, and certain medications such as intravenous contrast administration or antibiotics.
Most participants in this study were admitted to the nephrology and medicine department, which is consistent with findings from Teo et al.,1 Vasanth et al.,21 and Kiran et al.20 This indicates that medical rather than surgical issues are the predominant underlying causes of CA-AKI. However, it’s crucial to acknowledge that potential selection bias may arise from enrolling patients based on consultation referrals.
Most of our study participants were in KDIGO stage 3, followed by stage 2 and stage 1. Abebe et al.,13 Kaaviya et al.,2 Korula et al.,12 Chetlapalli et al.,14 and Bhadade et al.11 also reported stage 3 as the most common KDIGO stage at which CA-AKI diagnosis was made. However, some studies indicated stage 1 as more common.1,16 Methodological variations, patient enrollment approaches, and delays in reporting or hospital referral may contribute to these discrepancies. Our reliance on referral calls to nephrology may have resulted in missing milder AKI cases, and the limited availability of baseline creatinine data influenced severity classification.
In our study, a single etiology of AKI was noted in most patients, similar to Teo et al.’s findings.1 Our study reported sepsis as the most common cause, followed by shock, glomerulonephritis, and hypovolemia. This was also in accordance with other studies by Teo et al.1, Iram et al.10, Abebe et al.13, Chetlapalli et al.14 and Rathore et al.18 and Vasanth G et al.21 Overall, our study contributes valuable insights into the unique characteristics and outcomes of CA-AKI in a developing country context.
Our study delved into the etiology of sepsis based on the initial focus, revealing a diverse distribution across different organ systems. Gastrointestinal causes predominated, comprising 30.4% of cases, followed by urinary tract infections at 17.1%. A study by Kaaviya et al.2 indicated that 46.23% of 186 AKI patients had an infective cause, with pyelonephritis being the most common at 17.7%. Despite reported decreases in AKI incidence linked to acute diarrheal illness by Vivek Kumar et al.,22 our findings affirm the continued prominence of gastrointestinal infections as a common cause of AKI. This aligns with existing research, highlighting the persistent significance of these sources in septic conditions. The sequential prevalence of gastrointestinal and urinary tract infections underscores the need for increased clinical awareness and preventive strategies at the community level. Sepsis with an unspecified organ focus accounted for 16.5% of cases, emphasizing the challenges in identifying the origin in certain instances. Additionally, respiratory tract and skin involvement contributed to 13.9% and 13.3%, respectively, illustrating the diverse nature of septic presentations.
Our study highlights diverse drug contributors to renal impairment, with NSAIDs being the most prevalent (46.3%). This underscores the significant impact of NSAIDs on renal function, as supported by a study23 indicating a 58% increased risk of AKI with NSAID use, emphasizing the need for heightened awareness of renal risks in clinical practice. The varying contributions of alternative medications and anti-tubercular drugs underscore the importance of evaluating multiple factors when assessing and managing drug-induced renal impairment. This emphasizes the crucial need for clinicians to remain vigilant about the potential impact of different drug classes on renal function.
Our study revealed that most patients had complete recovery, followed by partial and non-recovery. González et al.8 and Iram et al.10 reported similar results in their studies. Vasanth et al.21 reported recovery in 53.5%, followed by end stage renal disease (ESRD), while Rathore et al.18 reported recovery in 35.2% and ESRD in 34.1% of patients. These findings underscore the reversible nature of AKI and the potential for prevention through early intervention and timely management.
The current study revealed several risk factors independently associated with mortality, including elderly age, multiple AKI-causing etiologies, hepatorenal syndrome, presence of shock, glomerulonephritis, sepsis, MV requirement, vasopressor use, comorbidities, diabetes, TLC, serum protein, and albumin. Similarly, Chetlapalli et al.14 found hypotension, anemia and the need for RRT associated with mortality. In a study by Abebe et al,13 hyperkalemia, sepsis, anemia, need for RRT, and age ≥60 years were found to be correlated with mortality. As per the results of Kaaviya et al.,2 hypotension, mechanical ventilation, thrombocytopenia, and anuria were significantly associated with death. Lee et al.16 found factors such as elderly age, heart failure, liver disease, diuretic use, pre-admission hemoglobin, albumin, and platelet count to be significantly associated with mortality. These factors emphasize the complexity and multi-faceted nature of AKI outcomes.
In our study, a mortality rate was reported in two-fifths (34.5%) of the patients who had CA-AKI. This was consistent with the findings of other studies: 34.6% by Chetlapalli et al.,14 42.9% by González et al.,8 30.7% by Rathore et al.,18 and 46% by Bhadade et al.11 However, variations in mortality rates have been observed in a few studies ranging from as high as 84.9% to as low as 4.4%.1,2,13,16,17,20 The difference in study design, site of admission (ICU vs general medical ward), underlying disease and comorbidities, and diverse causes of AKI may contribute to this variability. Monitoring AKI mortality is crucial because it signifies the severity of kidney dysfunction and the need for urgent medical intervention to prevent irreversible organ damage and death. Furthermore, monitoring AKI mortality rates helps healthcare systems identify areas for improvement in patient care and implement strategies to reduce mortality and improve overall outcomes. Diligent follow-up, especially in developing countries with limited research resources, is imperative for continuous knowledge pursuit and improvement in patient care outcomes.
The strength of our study lies in its exclusive focus on community-acquired AKI cases, providing an opportunity to alleviate the burden by appropriately managing the risk factors. Our study unveils diverse organ system involvement in sepsis etiology, emphasizing the importance of community-level preventive strategies and clinical awareness. The varied nature of septic presentations highlights the need for further research and advancements in sepsis management. The findings of the study indicating a higher mortality rate and progression of AKI to CKD in CA-AKI necessitate screening, comorbidities management, and early referral to improve the outcomes. Additionally, the 3-month follow-up duration identifies changing recovery patterns over time, contributing valuable insights for long-term care strategies and patient well-being.
Our study also had several limitations. As this was a single tertiary care center, short-term study, results cannot be generalized. Baseline creatinine was available in only a tiny percentage of patients. As our cohort had patients with underlying comorbidities and obstructive etiologies, the underlying CKD cannot be ruled out. In assessing the recovery pattern, we have used the empirical cut-off value of serum creatinine, which might have increased or decreased the AKI severity. As not all patients with AKI visiting the hospital are included, the early stage of AKI might be missed, contributing to selection bias. Hence, a prospective, multicentric study with long-term follow-up is required to enhance the reliability of the observed risk factors and outcomes in different geographical areas.
Conclusion
Our study on CA-AKI in a developing country underscores the predominance of younger patients, emphasizing the influence of infectious and environmental causes. Sepsis, particularly from gastrointestinal sources, emerges as a major contributor to CA-AKI, stressing the need for heightened clinical awareness and preventive strategies at the community level. NSAIDs, notably, present a significant risk for renal impairment, urging clinicians to be vigilant about potential renal impacts. The study highlights the reversible nature of AKI, with most patients experiencing complete recovery, yet mortality rates remain substantial, underscoring the severity and urgency of medical intervention.
Conflicts of interest
There are no conflicts of interest.
References
- A prospective study of clinical characteristics and outcomes of acute kidney injury in a tertiary care centre. BMC Nephrol. 2019;20:282.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Community acquired AKI: A prospective observational study from a tertiary level hospital in southern India. Indian J Nephrol. 2019;29:254-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Changing epidemiology of community-acquired acute kidney injury in developing countries: Analysis of 2405 cases in 26 years from eastern India. Clin Kidney J. 2013;6:150-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of acute kidney injury: How big is the problem? Crit Care Med. 2008;36:S146-151.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179-85.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury recovery pattern and subsequent risk of chronic kidney disease: An analysis of veterans administration data. Am J Kidney Dis. 2016;67:742-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012;60:402-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Survival and renal recovery after acute kidney injury requiring dialysis outside of intensive care units. Int Urol Nephrol. 2020;52:2367-77.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury recovery patterns in critically ill patients: Results of a retrospective cohort study. Crit Care Med. 2021;49:e683-92.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency of risk factors and outcome of hospital-acquired acute kidney injury. Cureus. 2020;12:e12001.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective study of acute kidney injury according to KDIGO definition and its mortality predictors. J Assoc Physicians India. 2016;64:22-8.
- [PubMed] [Google Scholar]
- Acute kidney injury-incidence, prognostic factors, and outcome of patients in an intensive care unit in a tertiary center: A prospective observational study. Indian J Crit Care Med. 2016;20:332-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mortality and predictors of acute kidney injury in adults: a hospital-based prospective observational study. Sci Rep. 2021;11:15672.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical profile and outcomes of acute kidney injury in intensive care units: A prospective single-center study. Ind J Kidney Dis. 2022;1:8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development and internal validation of a prediction model for hospital-acquired acute kidney injury. Clin Kidney J. 2021;14:309-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Predicting renal recovery after dialysis-requiring acute kidney injury. Kidney Int Rep. 2019;4:571-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The frequency and outcome of Acute kidney injury in a tertiary hospital: Which factors affect mortality?: Acute kidney injury and mortality. Artif Organs. 2015;39:597-606.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes and associated risk factors of post-hospitalization dialysis-dependent acute kidney injury patients. Nephron. 2017;137:105-12.
- [CrossRef] [PubMed] [Google Scholar]
- Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143-50.
- [CrossRef] [PubMed] [Google Scholar]
- Study of factors affecting the outcome in acute kidney injury (AKI) Int J Sci Stud. 2019;7:76-80.
- [Google Scholar]
- A study of factors determining the outcome of acute kidney injury patients requiring hemodialysis. Int J Res Med Sci. 2018;6:2974-82.
- [Google Scholar]
- Community-acquired AKI in Asia: An update. Semin Nephrol. 2020;40:456-67.
- [CrossRef] [PubMed] [Google Scholar]
- Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. Am J Epidemiol. 2000;151:488-96.
- [CrossRef] [PubMed] [Google Scholar]







