Translate this page into:
Collapsing glomerulopathy following anabolic steroid use in a 16-year-old boy with IgA nephropathy
Address for correspondence: Dr. Smita Mary Matthai, Department of Pathology, Central Electron Microscopy Unit, Wellcome Trust Research Laboratory, Christian Medical College, Vellore - 632 004, Tamil Nadu, India. E-mail: smitazach@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Collapsing glomerulopathy (CG) is a proliferative podocytopathy, increasingly recognized in a variety of disease conditions. We report a case of CG in a 16-year-old boy with IgA nephropathy (IgAN) who presented with acute kidney injury, marked proteinuria and hypertension following a short period of anabolic steroid use. Although CG has been associated with long-term anabolic steroid use among body builders, there is no data on the effect of anabolic steroid use in persons with underlying renal disease like IgAN. We postulate that development of CG in our patient could be temporally linked to intake of body-building steroids along with a predisposing background renal disease of IgAN.
Keywords
Anabolic steroids
collapsing glomerulopathy
human immunodeficiency virus-associated nephropathy
IgA nephropathy
Introduction
Collapsing glomerulopathy (CG) is a morphologically and clinically distinct podocytopathy characterized by segmental or global collapse of glomerular capillaries, formation of pseudocrescents and variable tubulointerstitial injury, presenting with an accelerated clinical course, not responding to standard therapeutic regimens.[123] Originally described in 1978 as an idiopathic disorder termed malignant focal segmental glomerulosclerosis (FSGS), it became a relatively frequent diagnosis of the 80s with the identification of human immunodeficiency virus-associated nephropathy (HIVAN), exhibiting a striking racial predilection for African Americans. In recent years, there has been a growing list of disorders associated with CG in non-HIV infected patients, which includes infections, autoimmune diseases,[4] intake of medicinal and illegal drugs,[567] and thrombotic microangiopathy (TMA). Conversely, the detection of CG in the setting of HIV has declined with the advent of antiretroviral therapy.[8] We report a case of CG associated with IgA nephropathy (IgAN), in a 16-year-old, non-HIV infected boy with a history of anabolic steroid use for enhancing his appearance.
Case Report
A 16-year-old boy presented with history of vomiting and headache of 15 days duration. On evaluation, he was found to have blood pressure of 160/80 mmHg. He had a history of consumption of anabolic steroids (dexona + dianabol [methandrostenolone] combination) for general body development for a duration of 3 months.
Laboratory studies revealed a serum creatinine of 2.27 mg/dl, urine protein to creatinine ratio of 6, proteinuria of 4.7 g in 24 h. Urine microscopy showed 10-12 white blood cells and 5-40 red blood cells per high power field. His liver function tests (LFT) were normal. There was no jaundice or evidence of cholestasis. Tests for antibodies to HIV, hepatitis B and C viruses were negative. There was no biochemical evidence of a TMA. Ultrasound examination of both kidneys revealed Grade II renal parenchymal changes.
The renal biopsy showed global glomerulosclerosis in four of the 15 glomeruli, with the remaining showing mesangial and endocapillary cell proliferation with paramesangial capillary wall thickening. The extra glomerular compartment showed interstitial fibrosis amounting to 20%, aggregates of mononuclear inflammatory cells, tubular atrophy, microcystic dilatation of tubules with hyaline casts and necrotic cells in nonatrophic tubular lumina [Figure 1].

- Implosive collapse of a glomerular tuft with poorly cohesive proliferating podocytes appearing to fall off into the increased urinary space. Periodic acid-Schiff (PAS) stain demonstrates protein resorption droplets in hypertrophic podocytes. Adjacent tubule shows microcystic dilatation (PAS, ×400)
The immunofluorescence (IF) done on two glomeruli revealed arborizing mesangial deposits of IgA (3+), IgG (2+ to 3+) and C3 (2+ to 3+) with extension into the paramesangial capillary walls. There was no staining for IgM, C1q or C4. Electron microscopic examination of seven glomeruli showed abundant paramesangial electron-dense deposits, focally extending subendothelially into peripheral capillary loops. Segmental thinning and splitting of glomerular basement membranes (GBMs) were present, suggestive of primary IgAN. One glomerulus showed segmental [Figures 2 and 3] and another showed global collapse with corrugated GBMs and pseudocrescent formation [Figures 4–6], features consistent with CG. Foot process effacement was extensive. Endothelial tubuloreticular inclusions were absent, in keeping with the patient's negative serology for HIV and other viral infections. There was no evidence of TMA.
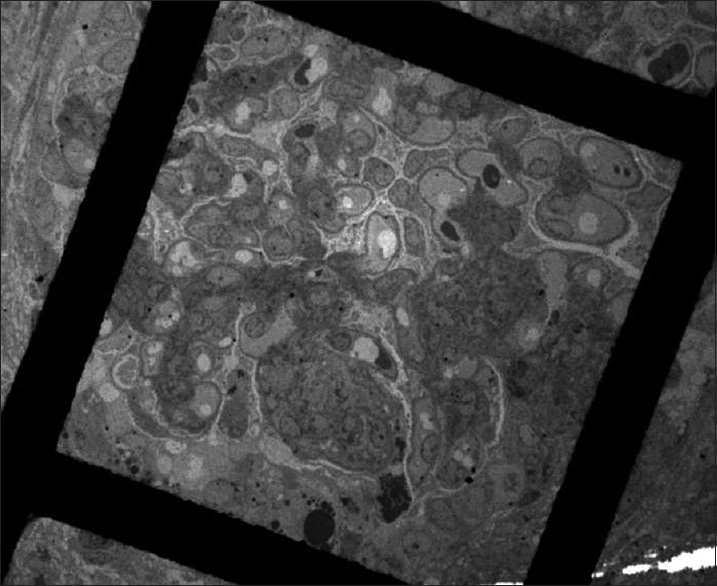
- Segmental collapse with overlying podocyte hyperplasia (transmission electron microscopy, ×390)
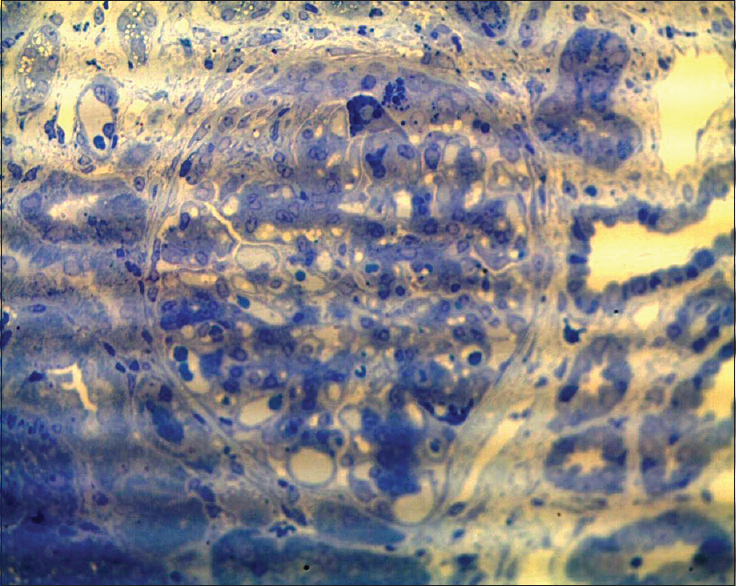
- Toluidine blue stain highlights protein resorption droplets in hypertrophied podocytes in a glomerulus with segmental collapse and early pseudocrescent (semi-thin sections, toluidine blue, ×400)

- Pseudocrescent in Bowman's space composed of proliferating podocytes, some with protein resorption droplets. Note the clear spatial demarcation from thin, nonproliferating layer of parietal epithelium. Abundant electron-dense deposits are present along with segmental wrinkling of glomerular basement membranes (transmission electron microscopy, ×610)

- Podocyte bridging and hyperplasia with underlying corrugated glomerular basement membranes in a glomerulus with collapsing glomerulopathy (transmission electron microscopy, ×1700)
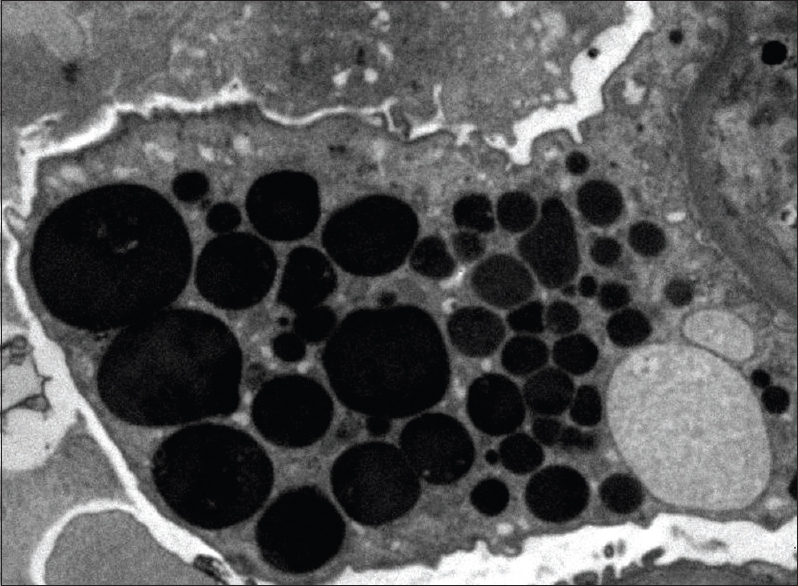
- Enlarged podocyte containing protein resorption droplets (transmission electron microscopy, ×6000)
The final diagnosis was CG associated with IgAN. The patient was treated with oral prednisolone and mycophenolate mofetil with therapeutic drug level monitoring and strongly recommended to abstain from further use of body-building drugs. On follow-up after 6 months, the patient has achieved partial remission with proteinuria decreasing to 1.6 g/24 h. But serum creatinine remained high at 2.96 mg/dl. The patient is continuing therapy and is on regular follow-up.
Discussion
Collapsing glomerulopathy is being increasingly reported in literature with growing awareness in the medical community of disorders other than HIV related to its occurrence. Although CG was considered to be a variant of FSGS in the Columbia classification, recent taxonomical categorization proposes CG as a podocytopathy distinct from FSGS.[9] CG is defined by presence of pseudocrescents in the Bowman's space, resulting from exuberant podocyte proliferation and associated collapse or wrinkling of GBMs whereas FSGS is characterized by podocytopenia.[910] Ultrastructurally, podocytes in CG are more cuboidal with loss of primary foot processes indicating a dysregulated phenotype as seen in this case Irrespective of etiology and clinical correlates,[12] CG has a poor prognosis, with rapidly deteriorating renal function and marked proteinuria refractory to standard therapy.
IgAN is the leading cause of glomerulonephritis globally with high incidence in Indian population[13] unlike CG, which is extremely rare in this population, with only occasional case reports in the setting of HIVAN and systemic lupus erythematosus.[14] This could be attributed to probable variations in genetic susceptibility, reflected in high prevalence of HIV and non-HIV associated CG among patients of African origin.[15] Although a recent study from France has reported occurrence of CG associated with IgAN (eight cases of CG among 128 IgAN cases), portending a poor prognosis, a definite etiological link has not been established.[1617] Another study from Portugal found concurrent mesangial IgA deposits in three cases of CG, one of which was in a hepatitis B-infected patient using illegal oral drugs.[18] Long-term abuse of anabolic steroids has been related to CG among body builders by Herlitz et al.[19] A direct nephrotoxic effect of anabolic steroids, in addition to post-adaptive stress driven by increased body mass was implicated in the pathogenesis of CG and FSGS in this study. Interestingly, seven patients who discontinued anabolic steroids achieved remission in contrast to one patient who did not abstain.[19]
The present case describes short-term anabolic steroid abuse and development of CG in a young patient from a low risk population with underlying primary IgAN. The diagnosis of primary IgAN was based on LM, IF and electron microscopy (EM) studies. EM demonstrated presence of paramesangial electron-dense deposits with GBM thinning and splitting, which are additional ultrastructural findings supportive of a primary rather than secondary IgAN. Normal LFT also made IgAN secondary to drug-induced hepatic dysfunction unlikely. The onset of CG followed intake of anabolic steroids and despite high prevalence of IgAN in this population, there is no previous documentation of concurrent CG and IgAN from India. There was no biochemical or histological evidence of TMA or severe ischemic changes or other viral infections to suggest possible etiologies of CG. Although all patients with IgAN exposed to anabolic steroids do not develop CG, the temporal sequence of events in our case, as described above, helps identify anabolic steroids as a co-factor precipitating CG in this patient.
Since mature podocytes do not normally enter the cell cycle unless subjected to strong mitogenic stimuli, accumulated stress due to repeated insults against a background of genetic susceptibility has been implicated in the pathogenesis of CG. This is probably applicable to our case also. Genetic analysis for CG could not be carried out on the patient. Nevertheless, this case supports the current school of thought that more than one hit to the podocyte may be involved in etiology of CG.
Conclusion
Because of its attendant poor prognosis, a high degree of clinical suspicion should entail careful search for CG on renal biopsies of all relevant cases, even in low incidence areas with high prevalence of other primary glomerulonephritis. This should be followed by an effort to ascertain the etiology, whenever possible, to rule out potentially modifiable causes like drug or toxin exposure. Finally, the alarming increase in anabolic steroid use for appearance enhancement, particularly among adolescents and youth, warrants physicians to target their efforts in counseling about potential nephrotoxic effects especially in the context of pre-existing renal disease.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int. 1994;45:1416-24.
- [Google Scholar]
- Idiopathic collapsing focal segmental glomerulosclerosis: A clinicopathologic study. Kidney Int. 1996;50:1734-46.
- [Google Scholar]
- Collapsing glomerulopathy in 19 patients with systemic lupus erythematosus or lupus-like disease. Clin J Am Soc Nephrol. 2012;7:914-25.
- [Google Scholar]
- Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164-72.
- [Google Scholar]
- Collapsing glomerulopathy following anthracycline therapy. Am J Kidney Dis. 2013;61:778-81.
- [Google Scholar]
- HIV-associated kidney glomerular diseases: Changes with time and HAART. Nephrol Dial Transplant. 2012;27:2349-55.
- [Google Scholar]
- A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2:529-42.
- [Google Scholar]
- Collapsing glomerulopathy associated with inherited mitochondrial injury. Kidney Int. 2008;74:237-43.
- [Google Scholar]
- Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int. 1999;56:2203-13.
- [Google Scholar]
- Characterization of kidney lesions in Indian adults: Towards a renal biopsy registry. J Nephrol. 2006;19:205-10.
- [Google Scholar]
- Collapsing glomerulopathy occurring in HIV-negative patients with systemic lupus erythematosus: Report of three cases and brief review of the literature. Lupus. 2011;20:866-70.
- [Google Scholar]
- Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841-5.
- [Google Scholar]
- Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int. 2011;79:643-54.
- [Google Scholar]
- Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. I. Immunohistochemical studies. Kidney Int. 2011;79:635-42.
- [Google Scholar]
- Collapsing glomerulopathy in Portugal: A review of the histological and clinical findings in HIV and non-HIV patients. Nephrol Dial Transplant. 2011;26:2209-15.
- [Google Scholar]
- Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol. 2010;21:163-72.
- [Google Scholar]







