Comparison of Urinary Beta-2 Microglobulin Levels in Children with SSNS and Calcineurin Inhibitor–Treated SRNS
Corresponding author: Dr. Mukta Mantan, Department of Pediatrics, Maulana Azad Medical College, University of Delhi, New Delhi – 110 002, India. E-mail: muktamantan@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yadav D, Mantan M, Mahajan B. Comparison of Urinary Beta-2 Microglobulin Levels in Children with SSNS and Calcineurin Inhibitor–Treated SRNS. Indian J Nephrol. 2024;34:149–54. doi: 10.4103/ijn.ijn_339_22
Abstract
Background:
While the utility of beta-2 microglobulin (β2M) has been explored in various renal conditions to identify tubulointerstitial damage, it has not been adequately studied in nephrotic syndrome. The primary objective of the study was to compare urinary β2M levels in children with steroid-sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS) in disease remission.
Materials and Methods:
This cross-sectional study was done at a tertiary care hospital between April 2019 and March 2020. Sixty children (2–18 years) with SSNS and SRNS (30 in each group) in remission were enrolled. SRNS patients were included after ≥1 year of treatment with calcineurin inhibitors (CNIs). Biochemical investigations were done to confirm remission; spot samples for urinary β2M were collected and estimation was done by an enzyme-linked immunosorbent assay (ELISA)-based kit.
Results:
Of the 60 children, 63% were boys. The median (interquartile range [IQR]) age at enrollment for SSNS and SRNS patients was 7 (4.1–9) and 11 (8.3–12) years, respectively. Urinary β2M levels were significantly higher in SRNS patients compared to SSNS patients (2.6 vs. 0.75 mg/ml, P < 0.0001). Patients who received cyclosporine for >2 years had higher median urinary β2M levels compared to those who received it for a shorter period (2.63 vs. 1.83 mg/ml, P = 0.03). Median β2M levels were higher in focal segmental glomerulosclerosis than minimal change disease (3.5 vs. 2.5 mg/ml).
Conclusion:
Urinary β2M levels were higher in SRNS compared to SSNS disease in remission, and β2M levels correlated well with CNI use of >2 years. It appears to be a promising noninvasive tool to identify early tubular damage and progression in patients with nephrotic syndrome, especially SRNS.
Keywords
Beta-2 microglobulin
calcineurin inhibitor
nephrotic syndrome
Introduction
Nephrotic syndrome in children is characterized by heavy proteinuria edema, proteinuria, hypoalbuminemia, and hyperlipidemia. More than 90% respond to oral steroids, and 60% patients require treatment with other immunosuppressants). Children with steroid-resistant disease are treated with calcineurin inhibitors (CNIs).1 These can cause nephrotoxicity on long-term use; and a renal biopsy is required to identify the tubulointerstitial damage.2 Concomitant use of other nephrotoxic drugs, diuretic use, and repeated episodes of acute kidney injury can aggravate tubulointerstitial damage.3,4
Enzymuria and low-molecular-weight proteinuria (e.g. beta-2 microglobulin, β2M) reflect tubular damage.5 With significant tubular damage, the urinary excretion of beta-2 microglobulin (β2M) increases; fractional excretion of β2M (FE-β2M) in urine is elevated in conditions with primary tubular defects like Fanconi’s syndrome, Wilson’s disease, cystinosis, etc.and so on and is also elevated in tubulointerstitial nephritis.6,7 The utility of serum and urinary β2M levels has been probed in patients with acute kidney injury, chronic kidney disease, and renal transplantation.8-10 β2M is a marker of poor prognosis in membranous nephropathy.10,11 Higher FE-β2M has been reported in children with tubular diseases when compared to those with glomerular conditions.12
Studies in children and adults with nephrotic syndrome have chiefly looked at the β2M levels in remission and relapse.13-17 A study comparing the β2M levels in children with steroid-sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS) enrolled patients with SRNS in relapse and reported no relationship to therapy.17 Higher urinary β2M values have been observed during relapses of nephrotic syndrome; however, they tend to normalize with remission. Persistenly elevated levels may indicate underlying tubular damage. In the present study, we explored the possibility of using urinary β2M level as a noninvasive marker to assess renal tubular damage in children with nephrotic syndrome, especially those on therapy with CNIs.
Materials and Methods
This cross-sectional study was conducted in the Department of Pediatrics of a tertiary care teaching hospital, from April 2019 to March 2020. The study was approved by the ethics committee of the institute, and written informed consent/assent was taken from the caregivers. All children and adolescents (2–18 years) with SSNS or SRNS (who were on at least 1 year of treatment with CNI) currently in complete remission (CR) were included. Children with congenital nephrotic syndrome, active urinary tract infection, acute kidney injury, or chronic kidney disease were excluded from the study. The primary objective of the study was to estimate urinary β2M levels in disease remission, and the secondary objectives were to determine the associations of urinary β2M levels with disease type, duration of disease, CNI use, and renal biopsy findings.
Standard definitions were used for describing nephrotic syndrome and its clinical course.18 A detailed history was taken and clinical examination done at the first visit to identify patients with SSNS and SRNS. Baseline information like age, diagnosis, duration of nephrotic syndrome, disease type, therapy received in the past, and renal biopsy findings were recorded. A urine protein dipstick examination was done to look for remission in both groups. CR was defined as presence of trace or negative dipstick proteinuria or spot urine protein to Cr ratio less than 0.2 mg/mg. Partial remission (PR) was defined as patients showing 1–2+ dipstick proteinuria or protein to Cr ratio from 0.2 to 2 mg/mg, serum albumin >3.0 g/dl, and no edema. Persistence of 3–4+ proteinuria or protein to Cr ratio >2, albumin less than 2.5 g/dl, or edema constituted nonresponse (NR).
Each patient underwent biochemical investigations (kidney function test, total protein, serum albumin, cholesterol level) for confirmation of remission, and 24-h urine collection was done for estimation of protein and Cr during CR. Estimated glomerular filtration rate (eGFR) was estimated by modified Schwartz equation.19
Estimation of β2M
As previous studies have shown that urinary β2M disintegrates at low urinary pH, children were given sodium bicarbonate tablets 2 mEq/kg/d in twice-daily dose starting 1 day before collection.20 Urine pH was checked for each collected urine sample (desired pH >6), and the samples were stored at −80°C till further testing. Urine β2M level estimation was done by an enzyme-linked immunosorbent assay (ELISA; Calbiotech # BM010T)-based kit. The 24-h values were calculated from urinary volume multiplied by the spot values of β2M.
Based on the sample size calculation for a cross-sectional study with the expected proportion of event (elevation of urinary β2M levels) in SRNS as 0.3 compared to 0 in the SSNS group, with a power of 80%, significance of 0.05, and a ratio of 1:1 in each group, the sample size calculated was 56 (28 in each group); hence, a total of 60 patients were enrolled.
Statistical analysis
Data was entered into a Microsoft Excel spreadsheet and analyzed using descriptive statistics and by Statistical Package for the Social Sciences (SPSS) IBM software version 24.0. The median and interquartile ranges (IQRs) were calculated for quantitative and percentages for categorical variables. The Chi-square test, Mann– Whitney U test, and Wilcoxon signed-rank test were used to compare groups with categorical data, and Student’s t-test or one-way analysis of variance (ANOVA) was used for comparing the association between groups with continuous data. For all comparisons, 5% probability (P < 0.05) was considered significant. Appropriate statistical tests for correlation (Pearson correlation) were applied, and receiver operating curves (ROC) were drawn for β2M levels in the SSNS and SRNS groups.
Results
A total of 60 children were enrolled; 30 had SSNS and another 30 SRNS. The median age (IQR) of the study population was 9 (7–11) years. In the SSNS group, seven (23%) children had frequent relapsing nephrotic syndrome (FRNS) and eight (26.7%) had infrequent relapsing nephrotic syndrome (IFRNS), while 15 (50%) had steroid-dependent nephrotic syndrome (SDNS) course. Of the 30 SRNS patients, six (20%) had initial resistance and 24 (80%) had late steroid resistance. Baseline and biochemical parameters were comparable between SSNS and SRNS patients [Table 1].
| Parameters | SRNS (n=30) | SSNS (n=30) | P |
|---|---|---|---|
| Age at enrollment (years) | 11 (8.3-12) | 7 (4.1-9) | <0.0001 |
| Median (IQR) | |||
| Age of disease onset (years) | 3.5 (3-6) | 3 (2-5.7) | 0.088 |
| Median (IQR) | |||
| Disease duration (years) | 5.5 (4-6.87) | 3 (1.5-4.75) | 0.0010 |
| Median (IQR) | |||
| Type of disease | Initial resistance 6 (20%) | SDNS 15 (83.3%) | |
| n (%) | Late resistance 24 (80%) | FRNS 7 (23.3%) | |
| IFRNS 8 (26.7%) | |||
| Biopsy finding (%) | MCD 18 (60.0%) | ||
| FSGS 9 (30%) | |||
| C3GN 2 (6.7%) | |||
| MesPGN 1 (3.3%) | |||
| Alternative agents besides prednisolone | Cyclosporine only- 16 (53.3%) | MMF- 11 (36%) | |
| Tacrolimus only- 4 (13.3%) | Levamisole- 9 (30%) | ||
| Both drugs- 10 (33.3%)a | Cyclophosphamide - 7 (23%) | ||
| Cyclosporine | |||
| ≤2 years- 10 (33%) | |||
| >2 years- 16 (53%) | |||
| Tacrolimus | |||
| ≤2 years- 12 (40%) | |||
| >2 years- 2 (6%) | |||
| GFR in ml/min/1.73 m2 | 111.9 (101.8-143.9) | 125.1 (110.3-148.6) | 0.077 |
| Median (IQR) | |||
| Serum creatinine (mg/dl) | 0.6 (0.5-0.7) | 0.5 (0.4-0.58) | 0.0007 |
| Total serum protein (g/dl) | 6.6 (6.2-7.2) | 6.5 (5.9-6.7) | 0.051 |
| Serum albumin (g/dl) | 3.85 (3.4-4) | 3.8 (3.5-4) | 0.655 |
| Serum cholesterol (mg/dl) | 182 (146.8-198.3) | 176 (154.5-193.3) | 0.771 |
| Serum bicarbonate (mEq/l) | 23.2 (22.3-24.9) | 23 (22-23.7) | 0.378 |
FRNS=frequent relapsing nephrotic syndrome, GFR=glomerular filtration rate, IFRNS=infrequent relapsing nephrotic syndrome, IQR=interquartile range, SDNS=steroid-dependent nephrotic syndrome, SRNS=steroid-sensitive nephrotic syndrome, SSNS=steroid-sensitive nephrotic syndrome. aPatients received a change in CNI due to adverse event or nonresponse. Text in bold indicates significant P values
A comparison of 24-h urinary parameters of SRNS and SSNS patients did not reveal any significant difference in either group, except proteinuria, which was more in the SRNS group than the SSNS group (P = 0.002) [Table 2]. The median urinary spot β2M level for the SRNS group was 2.6 mg/ml, at least 3 times higher than the SSNS group (0.75 mg/ml, P < 0.0001). Two patients had a urine β2M level of 10 mg/ml. Similarly, the spot β2M/Cr ratio and median 24-h urine β2M values were higher in the SRNS group (P < 0.0001).
| Urine parameter | SRNS Median (IQR) | SSNS Median (IQR) | P |
|---|---|---|---|
| Urine creatinine (mg/dl) | 38.9 (29.3-54.5) | 32.5 (24-46.5) | 0.126 |
| 24-h urine protein (mg/d) | 199 (126-374) | 107 (82-158) | 0.002* |
| Urine β2M (µg/ml) | 2.6 (1.6-3.8) | 0.75 (0.54-1.23) | <0.0001* |
| Spot β2M/creatinine ratio (µg/g) | 0.06 (0.03-0.09) | 0.02 (0.01-0.03) | <0.0001* |
| 24-h urine β2M (µg) | 3250 (1237-5760) | 525 (385-780) | <0.0001* |
β2M=beta-2 microglobulin, IQR=interquartile range, SRNS=steroid-sensitive nephrotic syndrome, SSNS=steroid-sensitive nephrotic syndrome. *P<0.05 significant
Spot urinary β2M values in different types of nephrotic syndrome are provided in Table 3. Among SSNS children, the median β2M values were numerically higher in the FRNS patients than the SDNS and IFRNS patients (P = 0.302). In SRNS children, the median β2M values were higher in the patients with initial resistance compared to those with late resistance, though the difference was not statistically significant (P = 0.062). The median β2M values were compared in different biopsy types in children with SRNS and the levels were found to be higher in FSGS disease than minimal change disease, but the difference was not statistically significant (P = 0.413).
| Categories | β2M value (µg/ml) Median (IQR) | P |
|---|---|---|
| SDNS | 0.7 (0.485-1.05) | 0.302 |
| FRNS | 1.25 (0.65-1.35) | |
| IFRNS | 0.7 (0.465-0.977) | |
| SRNS | ||
| Initial resistance | 4.08 (2.64-6.64) | 0.062 |
| Late resistance | 2.55 (1.44-3.71) | |
| Biopsy findings | ||
| MCD | 2.45 (1.52-3.80) | 0.413 |
| FSGS | 3.5 (1.05-5.97) | |
| C3GN | 2.65 (2.50-2.80) | |
| MesPGN | 3.5 (2.05-2.5) | |
| Cyclosporine duration | ||
| ≤2 years (n=10) | 1.83 (0.98-3.53) | 0.415 |
| >2 years (n=16) | 2.63 (2-4.65) | |
| Tacrolimus duration | ||
| ≤2 years (n=12) | 3.60 (2.57-4.22) | 0.296 |
| >2 years (n=2) | 2.0 (1.2-2.8) |
β2M=beta-2 microglobulin, FRNS=frequent relapsing nephrotic syndrome, IFRNS=infrequent relapsing nephrotic syndrome, IQR=interquartile range, SDNS=steroid-dependent nephrotic syndrome, SRNS=steroid resistant nephrotic syndrome, MCD=minimal change disease, FSGS=focal segmental glomerulosclerosis, C3GN=C3 glomerulonephritis, MesPGN=Mesangial proliferative glomerulonephritis
Upon correlating the urinary β2M levels with duration of CNI use, we found a positive correlation between duration of cyclosporine use and β2M levels (r = 0.41, P = 0.03) [Figure 1], though this was not significant in case of tacrolimus (r = 0.002, P = 0.9) [Figure 2].
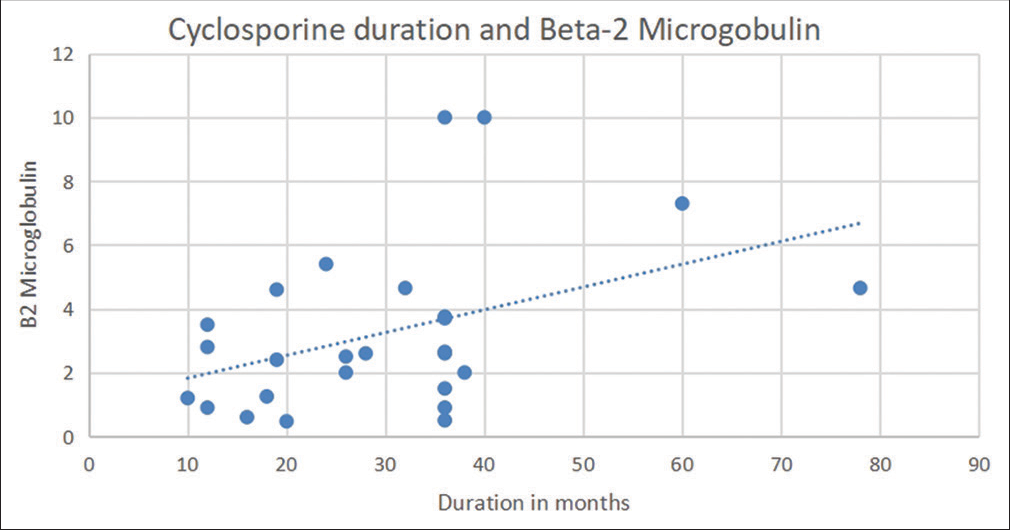
- Correlation between urine β2M levels and duration of cyclosporine use. β2M = beta-2 microglobulin.
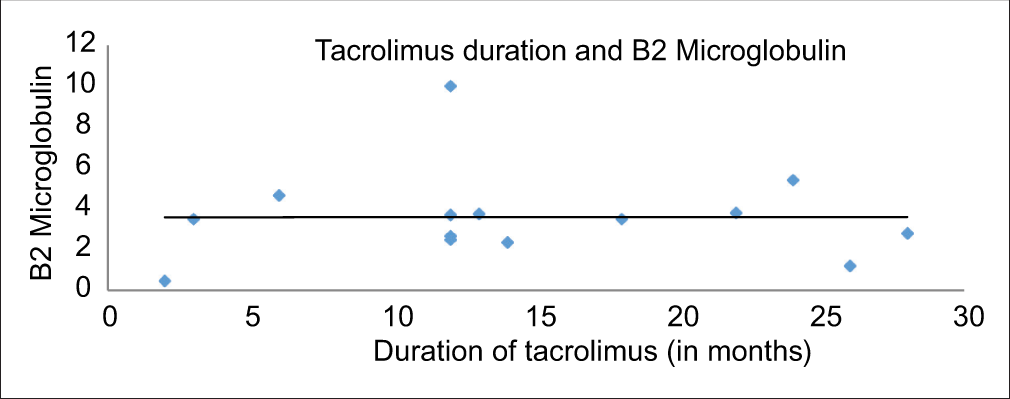
- Correlation between urine β2M levels and duration of tacrolimus use (n = 14). β2M = beta-2 microglobulin.
Also, a positive correlation was found between serum Cr (r = 0.5) and β2M levels in the SRNS group, while no correlation was found in the SSNS group. The 24-h urine protein excretion showed significant positive correlation (r = 0.57) with urinary β2M values in SRNS children, while no correlation was seen in SSNS children. According to the ROC curve (area under the curve [AUC] 0.882), a cutoff of 1.35 mg/ml for urine β2M clearly differentiated between SRNS and SSNS with a sensitivity of 76% and specificity of 90%.
Discussion
CNI-induced nephrotoxicity is a major challenge in the treatment of SRNS. As renal biopsy is an invasive procedure, there is need to look for noninvasive biomarkers to identify early tubular damage in SRNS.
β2M, a low-molecular-weight (11,800 kD) plasma protein is easily filtered and almost 99.9% is reabsorbed by the kidney and catabolized by proximal tubular cells.5,6 Normal serum value of β2M is 0–2 mg/ml, and the normal urinary excretion is 0–0.3 mg/ml or 0.3 mg/.21 β2M has been identified previously as a marker of tubular damage in other renal conditions like acute kidney injury (AKI), renal transplant, chronic kidney disease, and diabetic nephropathy.7,8,22
In the study there were 36.7% children with FRNS/SDNS and 26.7% IFRNS; mycophenolate mofetil was the most commonly used alternative agent followed by levamisole and cyclophosphamide amongst SSNS. In the SRNS group all patients received CNI’s and about 63.3% of the patients had received cyclosporine or tacrolimus for more than two years at the time of evaluation. Calcineurin inhibitors are usually used for a period of 2-3years for treatment of SRNS disease.23 The biopsy findings showed that 60% of SRNS was due to MCD and 30% due to FSGS; the incidence of MCD was higher compared to previous studies.24,25 This could be due to a larger proportion of late resistance (80%) in the present cohort and inclusion of patients in complete remission which is more likely to be associated with MCD.
In the present study, the median urinary β2M level in the SRNS group was three times higher than the SSNS group, and this difference was statistically significant, indicating more tubular damage. Similarly, the spot urinary β2M/Cr ratios and the 24-h urine β2M values too were elevated in the SRNS group compared to the SSNS group. Median β2M levels were higher in those with initial resistance in comparison to those with late resistance, but the difference was not statistically significant. Median β2M levels were higher in FRNS cases in comparison to SDNS and IFRNS cases, but the difference was not statistically significant. A study conducted in 37 idiopathic nephrotic syndrome patients (24 SSNS and 13 SRNS) showed that urinary β2M levels were high in 75% of patients with idiopathic nephrotic syndrome before treatment; SRNS patients had a higher value of median urinary β2M levels than SSNS patients (P < 0.01) at the initial evaluation. In the SSNS group, urine β2M decreased significantly after remission, but it remained high in the SRNS group.13 Similarly, a study in children with nephrotic syndrome found that the urinary β2M levels were higher in SRNS and SSNS patients in relapse than in SSNS patients in remission and controls (P < 0.0001).14 Other pediatric studies also showed higher values of β2M during relapse; however, the groups were heterogeneous and small and patients were compared during both relapse and remission.15,16 Our study was chiefly aimed to see if β2M can be used as a marker of chronic tubular damage once the patients are in remission and with normal glomerular functions, as heavy proteinuria during relapse would also elevate the β2M levels, which may not have long-term consequences. While the SRNS patients had a disease period longer than SSNS children (5.5 vs. 3 years), it is less likely to influence the results as most patients had late resistance and were started on CNIs promptly after the diagnosis and only those patients who were in CR were enrolled.
A positive correlation between duration of cyclosporine use and urinary β2M levels suggests that these levels could be used to identify early toxicity. As the SRNS patients were enrolled after a minimum duration of 1 year of CNI use, the higher levels of β2M are possibly reflective of tubular damage due to the drug, though this observation needs confirmation in prospective studies.
The primary limitation of the study is its one-time cross-sectional nature and that the baseline values of β2M were not available. Also, re-biopsy findings for calcineurin toxicity were not available for most patients. Simultaneous urinary β2M levels and biopsy finding might have given a better insight into the utility of β2M for diagnostic purpose. A larger sample size and prospective studies with baseline and follow-up values of β2M would help in confirming these findings further in future. However, a major strength of the study is that the population enrolled was homogeneous as all patients were in remission and all SRNS patients had received CNIs for at least 1 year. None of the previous studies have tried to correlate the therapy of SRNS to β2M levels. As the use of these drugs is increasing for treatment of SRNS, there is a need to explore noninvasive markers for early identification of tubulointerstitial damage.
Conclusion
Children with SRNS had higher values of urinary β2M than the children with SSNS (both in disease remission), and urinary β2M may be used as a marker to identify early tubulointerstitial damage. Further studies are required to validate this biomarker in more diverse patient population to determine its true clinical diagnostic and prognostic value, especially in children receiving calcineurin treatment.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Update on the treatment of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2022;37:303-14.
- [CrossRef] [Google Scholar]
- Calcineurin inhibitor induced nephrotoxicity in steroid resistant nephrotic syndrome. Indian J Nephrol. 2013;23:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481-508.
- [CrossRef] [PubMed] [Google Scholar]
- Calcineurin inhibitor nephrotoxicity: A review and perspective of the evidence. Am J Nephrol. 2013;37:602-12.
- [CrossRef] [PubMed] [Google Scholar]
- Low molecular weight proteins and the kidney: Physiologic and pathologic considerations. Ultrastruct Pathol. 1994;18:89-98.
- [CrossRef] [PubMed] [Google Scholar]
- Beta 2-microglobulin: Its significance in the evaluation of renal function. Kidney Int. 1987;32:635-41.
- [CrossRef] [PubMed] [Google Scholar]
- Rediscovering Beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med (Lausanne). 2017;4:73.
- [CrossRef] [PubMed] [Google Scholar]
- Cystatin C and Beta 2-microglobulin: Markers of glomerular filtration in critically ill children. Crit Care. 2007;11:R59.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82:1297-303.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of b2-microglobulin as a urinary biomarker for chronic allograft nephropathy using proteomic methods. Proteomics Clin Appl. 2011;5:422-31.
- [CrossRef] [PubMed] [Google Scholar]
- Beta-2-microglobulin is superior to N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2008;23:2546-51.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Beta-2 microglobulin to diagnose tubulointerstitial renal lesions in children. Kidney Int. 1986;30:91-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of steroid responsiveness in idiopathic nephrotic syndrome using urinary retinol binding protein and Beta-2 microglobulin. Ann Inter Med. 1992;116:905-9.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary-N-acetyl-beta-D glucosaminidase and Beta-2 microglobulin excretion in primary nephrotic children. Nephron. 1996;74:401-4.
- [CrossRef] [PubMed] [Google Scholar]
- Follow-up of steroid resistant nephrotic syndrome: Tubular proteinuria and enzymuria. Pediatr Nephrol. 2000;15:252-8.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary beta-2 microglobulin and lysozyme in nephrotic syndrome. Indian J Nephrol. 2005;15:84-90.
- [CrossRef] [PubMed] [Google Scholar]
- Tubular injury in children with steroid-resistant nephrotic syndrome. Am J Clin Med Res. 2019;7:9-13.
- [Google Scholar]
- Management of steroid sensitive nephrotic syndrome: Revised guidelines. Indian Paediatr. 2008;45:203-14.
- [Google Scholar]
- New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629-37.
- [CrossRef] [PubMed] [Google Scholar]
- Beta-2 Microglobulin instability in pathological urine. Clin Chem. 1982;28:1330-3.
- [CrossRef] [Google Scholar]
- The serum levels and urinary excretion of b2-microglobulin in apparently healthy subjects. Scand J Clin Lab Invest. 1972;29:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical utility of serum beta-2 microglobulin as a predictor of diabetic complications in patients with type 2 diabetes without renal impairment. Diabetes Metab. 2014;40:459-65.
- [CrossRef] [PubMed] [Google Scholar]
- IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35:1529-61.
- [CrossRef] [PubMed] [Google Scholar]
- Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55:1885-90.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern of steroid resistant nephrotic syndrome in children living in the kingdom of Saudi Arabia: A single center study. Saudi J Kidney Dis Transpl. 2009;20:854-7.
- [Google Scholar]







