Translate this page into:
Crescentic Glomerulonephritis in Human Immunodeficiency Virus Infection
Corresponding author: Jasmine Sethi, Department of Nephrology, PGIMER, Chandigarh, India. E-mail: jasmine227021@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vijayakumar NA, Bhansaly P, Sethi J, Nada R, Shekhar A. Crescentic Glomerulonephritis in Human Immunodeficiency Virus Infection. Indian J Nephrol. 2024;34:663-4. doi: 10.25259/ijn_553_23
Abstract
The spectrum of kidney disease among human immunodeficiency virus (HIV) infected patients is extensive. We describe a young male who was recently detected with HIV infection and antineutrophil cytoplasmic antibody (ANCA) negative pauci-immune crescentic glomerulonephritis. The patient had no extrarenal vasculitis involvement. Patient was successfully managed with highly active antiretroviral therapy (HAART) and oral steroids. In our case report, we have also reviewed the literature of crescentic glomerulonnephritis in HIV infection.
Keywords
HIV
PLHA
Crescentic
Pauci-immune
Glomerulonephritis
Renal
Introduction
The spectrum of kidney disease among patients infected with the human immunodeficiency virus (HIV) is extensive.1 Crescentic glomerulonephritis is uncommon in HIV patients and can be due to HIV Associated Immune Complex Kidney Disease (HIVICK) (lupus-like full-house pattern), crescentic IgA nephropathy, Cytomegalovirus (CMV) infection, Epstein-Barr virus (EBV) infection, anti-glomerular basement membrane (GBM) disease, and pauci-immune glomerulonephritis.2,3 Antineutrophil cytoplasmic antibody (ANCA) positivity has been detected in HIV patients with a prevalence between 13% and 83%, without evidence of clinical vasculitis and with a poor overall prognosis.4
Case Report
We describe a young male in his third decade with no previous comorbidities, who presented with complaints of easy fatiguability, shortness of breath, cola-colored urine, and decreased urine output for 1 week. There was no fever, skin rash, sore throat, joint pains, weight loss, or intake of alternative medicines. The patient denied history of recreational drug abuse, but had exposure to commercial sex workers in the last 1 year. Laboratory parameters revealed hemoglobin of 10.3 g/dl, platelet count of 270,000/mm3, total leukocyte count of 7400 cells/mm3, serum creatinine of 4 mg/dl, and serum albumin of 3 g/dl. Urinalysis revealed albumin 2+ and 10–15 red blood cells (RBCs) per high-power field (granular RBC casts), with 24-h urine protein estimation of 2.2 g/day. Ultrasonography revealed normal sized kidneys. The patient was detected to have positive HIV serology by enzyme-linked immunosorbent assay (ELISA) and western blot, with a CD4 count of 413 cells/µl. A kidney biopsy revealed 10 glomeruli, out of which six showed crescents (one cellular, three fibrocellular, and two fibrous) [Figure 1]. Direct immunofluorescence showed no deposits of immunoglobulins or complements. Antinuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (anti-myeloperoxidase [anti-MPO] and anti-proteinase 3 [anti-PR3]), serum C3 and C4, cryoglobulins, rheumatoid factor, hepatitis B/C/CMV/EBV serology, and anti-glomerular basement membrane antibody (EIA) were negative. The patient was started on highly active antiretroviral therapy (HAART) with abacavir, lamivudine, and dolutegravir and oral steroids in a dose of 0.5 mg/kg. At 1-month follow up, patient’s serum creatinine had decreased to 2.4 mg/dl and steroids were tapered.
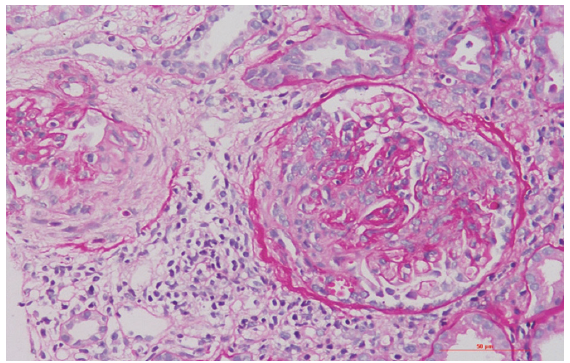
- Renal biopsy showing two glomeruli with fibrocellular crescents and focal rupture of the Bowman capsule (periodic acid Schiff stain, 200×).
Discussion
We conducted a search on PubMed and Scopus databases from 1996 to June 2023 using the following terms: “Renal involvement in HIV,” “Renal involvement in PLHA patients,” “Crescentic glomerulonephritis,” “Rapidly progressive renal failure in HIV patients.” We included case reports on crescentic glomerulonephritis with renal dysfunction in HIV patients. A total of 10 cases of crescentic glomerulonephritis in HIV patients were identified, including our case. The demographics, clinical and laboratory parameters are summarized in Table 1. Average age at presentation was 37.5 years (standard deviation [SD] 9.5), and males constituted the majority (two thirds). African American and other races were equally represented. Majority had severe renal insufficiency (mean serum creatinine of 9.9 mg/dl), with around 50% requiring renal replacement therapy. All patients had significant proteinuria with a mean of 3.9 g/24 h. The known HIV duration before the onset of crescentic glomerulonephritis was 5.6 ± 6.0 years, with three patients detected to have HIV at the time of renal biopsy. Three patients had positive ANCA serologies, one had positive anti-GBM antibody serology, and one had positive ANA. Three patients had concurrent viral infection – one with Epstein barr virus (EBV) infection documented in the mediastinal nodes, one with Cytomegalovirus (CMV) intranuclear inclusions in the renal tubular cells, and one patient with chronic hepatitis B. Pauci-immune crescentic glomerulonephritis was predominant, accounting for 40% of cases (including our case). Among these cases, 50% (two out of four) displayed positive anti-MPO antibodies and one patient had evidence of Cytomegalovirus (CMV) infection in the form of intranuclear inclusions in the renal tubular cells. Infection-related causes contributed to 30% of cases, encompassing one patient each with Cytomegalovirus (CMV), Epstein barr virus (EBV), and Salmonella typhimurium-associated cases. One patient had lupus-like immune complex glomerulonephritis with negative ANA/anti–double-stranded DNA (dsDNA). All patients received HAART, 40% of patients received cyclophosphamide, while 10% received rituximab. In most cases, steroid therapy was given (80%, n = 8). Plasmapheresis was utilized in one patient with concurrent anti-GBM positivity. Four out of 10 patients had clinical response either in the form of reduction in serum creatinine or dialysis independency. Four patients remained dialysis dependent and one patient expired.
| Baseline variable | Result |
|---|---|
| Known duration of HIV (years), mean ± SD | 5.6 ± 6.0 |
| Race (African American/other) | 5/5 |
| Hematuria | 10 (100%) |
| Oliguria | 4 (40%) |
| Hypertension | 5 (50%) |
| Dialysis dependent | 5 (50%) |
| Serum creatinine (mg/dl) | 9.98 ± 9.90 |
| Estimated GFR (ml/min/1.73 m2) | 14.1 ± 13.25 |
| Serum albumin (g/dl), mean ± SD | 2.42 |
| 24-h urine protein (g/day) | 3.95 |
| Hemoglobin (g/dl) | 9.62 |
| CD4 count (cells/µl) | 301.1 ± 137.76 |
| Positive anti-MPO or anti-PR3 | 3 (30%) |
| Positive anti-GBM | 1 (10%) |
| Positive ANA/dsDNA | 1 (10%) |
| Low C3 (positivity number, %) | 1 (10%) |
| Positive viral serology | 3 (30%)- one each for EBV/CMV and hepatitis B |
ANA: Antinuclear antibody, anti-MPO/-PR3: Anti-myeloperoxidase/anti-proteinase 3, dsDNA: Anti–double-stranded DNA, GFR: Glomerular filtration rate, HIV: Human immunodeficiency virus, SD: Standard deviation, EBV: Epstein barr virus, CMV: Cytomegalovirus , anti-GBM: Anti-glomerular basement membrane.
Mostly, pauci-immune crescentic glomerulonephritis is reported in association with positive ANCA serologies, although our patient had a negative serology.3 In addition to ANCA, secondary vasculitis due to opportunistic infections like Cytomegalovirus (CMV) and Epstein barr virus (EBV) should be considered in the differentials.4-6 Mainstay of treatment is HAART along with immunosuppression in selected patients.
If the clinical manifestations resemble vasculitis (with renal and pulmonary involvement) with positive ANCA serology, immunosuppressive treatment is administered, including steroids, cyclophosphamide, or rituximab.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Renal disease in human immunodeficiency virus-not just HIV-associated nephropathy. Indian J Nephrol. 2012;22:98-102.
- [CrossRef] [PubMed] [Google Scholar]
- Crescentic membranoproliferative glomerulonephritis in HIV infection; a mini-review with case study. J Nephropathol. 2022;11:e9842.
- [Google Scholar]
- ANCA-associated vasculitis and pauci-immune glomerulonephritis in HIV disease. BMJ Case Rep. 2014;2014:bcr2013202423. doi: 10.1136/bcr-2013-202423
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antineutrophil cytoplasmic autoantibody associated systemic vasculitis is associated with epstein-barr virus in the setting of HIV infection. Infect Dis Clin Pract (Baltim Md). 2013;21:50-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Crescentic IgA nephropathy and acute renal failure in an HIV-positive patient with enteric salmonella infection. Nephrol Dial Transplant. 1996;11:2320-3.
- [CrossRef] [PubMed] [Google Scholar]
- Renal vasculitis with HIV seropositivity: Potential manifestation of cytomegalovirus infection. Am J Kidney Dis. 1997;30:428-32.
- [CrossRef] [PubMed] [Google Scholar]







