Translate this page into:
Crystal-Induced Lower GI Necrosis in a Posttransplant Recipient with Diverticular Disease
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We report the case of a 67-year-old male kidney transplant recipient for 12 years with sodium polystyrene sulfonate crystal-induced ileocecal colitis. He had adult polycystic kidney disease with associated colonic diverticular disease. Here, we describe how a potentially fatal complication of colonic perforation was averted with appropriate investigations and management.
Keywords
Hyperkalemia
ileocecal colitis
sodium polystyrene sulfonate crystals
Introduction
Posttransplant gastrointestinal (GI) complications including infectious diarrheas, parasitic infestations, GI bleed, malignancies, diverticular disease, and inflammatory lesions. Sodium polystyrene sulfonate (SPS; Kayexalate), a cation-exchange resin, has been traditionally used in the treatment of hyperkalemia in chronic kidney disease and dialysis patients. SPS is usually administered as a suspension in water orally and rarely as a rectal enema.[1] Colonic necrosis is a rare complication of SPS therapy. A retrospective study at Rhode Island Hospital of 29 patients with SPS crystals in their pathology specimens demonstrated 11 patients with SPS-induced intestinal necrosis, and four out of the 11 patients had end-stage kidney disease. Several case studies across the world have reported the incidence of intestinal obstruction (some even due to bezoar formation) and necrosis following the administration of SPS without sorbitol.[2] Here, we describe the case of a 67-year-old male kidney transplant recipient due to adult polycystic kidney disease who was inadvertently consuming SPS without sorbitol for mild hyperkalemia leading to severe lower abdominal pain and ileocecal crystal deposits with necrosis.
Case Summary
A 67-year-old male, posttransplant recipient in 2008 with polycystic kidney disease presented to us with complaints of severe lower abdominal pain and constipation for the past 2 to 3 months. He had undergone bilateral native kidney nephrectomy and coronary artery bypass graft in 2012. He was a nonsmoker and was on regular follow-up with the cardiologist. He was diagnosed with diverticular disease in 2015. His immunosuppression consisted of prednisolone 5 mg, cyclosporine 50 mg bid (twice daily), and mycophenolate mofetil (MMF) 360 g bid. His baseline serum creatinine was 1.8 mg/dL, and drug levels of cyclosporine were maintained between 83 and 85 ng/mL. He was not on any potassium-retaining medications. He was having multiple episodes of watery diarrhea on and off since his cholecystectomy in 2014. He was intermittently ingesting 15 g SPS sachets (mixed in water) twice per day for many months, supervised elsewhere, for mild hyperkalemia in the past 3 years. On physical examination, he had mild tenderness in the hypogastric and left iliac fossa region with normal bowel sounds. The rest of the examination was unremarkable. Investigations showed hemoglobin 7.8 g/dL, white blood cell count 12,300 cells/mm3, platelets 260,000/mm3, neutrophils 86.7%, monocytes 2.3%, lymphocytes 10.8%, basophils 0.1%, eosinophils 0.1%, serum creatinine 1.95 mg/dL, potassium 5.58 mEq/L, bicarbonates 15 mEq/L, and magnesium 0.8 mg/dL. Urine analysis was normal. The stool occult blood test was positive. COVID-19 (coronavirus disease 2019) screening was negative. Computed tomography (CT) abdomen revealed multiple diverticula in the sigmoid and descending colon. The gastroenterologist did a colonoscopy and identified ileocecal and ascending colonic erythema, colonic angioectasia, and colonic diverticulosis [Figure 1]. Biopsy showed proximal colonic and ileocecal necrosis, possibly secondary to the deposition of purple-colored SPS crystals with a typical fish scale appearance [Figure 2]. The histology specimen did not show any amoeba, other ova/cysts, evidence of caseating granuloma, any features of MMF-induced colitis like crypt apoptosis and lamina propria eosinophilia, or cytomegalovirus (CMV) inclusions. Given the onset of symptoms after crystal consumption and the pathognomic SPS crystals in the biopsy, the patient was diagnosed with crystal-induced colitis after ruling out other possible causes. He was told to stop ingesting SPS, and his symptoms started resolving. His metabolic acidosis was corrected, and he continues to be on sodium bicarbonate. He was transfused with one unit of packed cells as his iron and vitamin B12 status were normal. Mild laxatives were prescribed for chronic constipation. Dietary counseling was given, and the patient was discharged and is on regular follow-up. No changes to his immunosuppression schedule were made, and his serum creatinine returned to baseline. This patient highlights the importance of multidisciplinary approach in appropriate diagnosis and management.

- Gastrointestinal biopsy
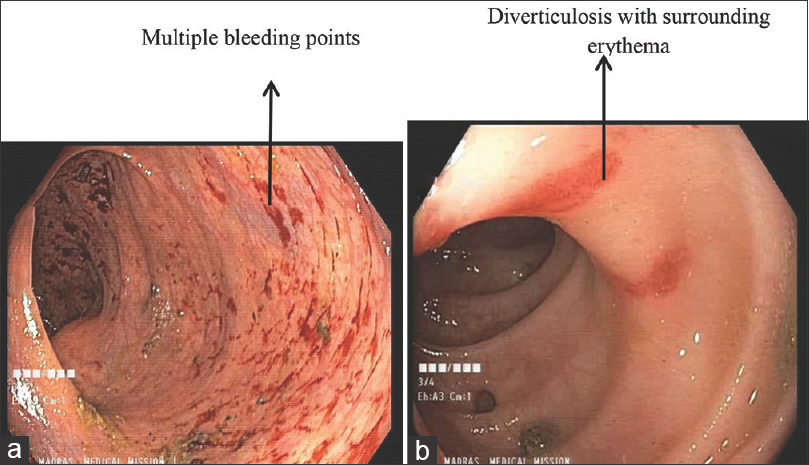
- Colonoscopy
Discussion
SPS crystal-induced lower GI necrosis still remains an underrecognized entity associated with significant morbidity and mortality, especially in patients on long-term immunosuppressive therapy as in our case. Risk factors for colonic perforation with the use of SPS include uremia, hypovolemia, hypotension, coagulation disorders, and immunosuppression. However, our patient was managed appropriately, thereby preventing the development of an acute abdomen.
SPS is an insoluble, synthetic, cation-exchange resin, resembling a crystalline lattice. SPS moves through the GI tract, exchanging sodium for potassium ions. The potassium bound SPS traverses through the colon and is eliminated in the feces.[3] It is not absorbed into the systemic circulation, and hence systemic bioavailability is unremarkable. Another case report demonstrated SPS (without sorbitol)-mediated small-bowel necrosis in a patient with graft versus host disease, despite no record of SPS administration more recently than 1 year prior for hyperkalemia.[4] The use of SPS should be limited because of the adverse GI side effects such as constipation and diarrhea, dyselectrolytemia including sodium loading, hypomagnesemia, hypocalcemia, and intestinal ischemia. SPS also enhances the risk of hypokalemia, and hence electrocardiogram changes (QT prolongation, U waves) and cardiac arrhythmias should be watched for.[5] In addition, serum potassium levels should be monitored for up to 24 hours after the initiation of treatment.
Posttransplant complications affecting the GI tract such as viral infections (CMV, herpes simplex), parasitic infestations (Strongyloides stercoralis, microsporidia), Clostridioides difficile colitis, MMF-induced toxicity, and GI malignancies were ruled out in our patient. The biopsy picture revealed mucosal injury, denuded epithelium, focally thickened subepithelial collagen band (>10 microns), and prominent purple-colored SPS crystals with a typical fish scale appearance. The correct diagnosis of crystal-induced ileocecal colitis was possible by CT abdomen followed by the colonoscopy studies ruling out colonic diverticulitis, which was a differential diagnosis in this patient.
Our patient with mild hyperkalemia, which was not life-threatening, received SPS without supervision, when correction of metabolic acidosis would have ameliorated the hyperkalemia. Hence, in mild to moderate hyperkalemia, resolution with intravenous fluids, correction of metabolic acidosis, diuretics, and dietary modifications should be attempted before prescribing the SPS agent. In emergency cases, alternative therapies such as intravenous calcium, intravenous insulin, and inhaled beta-adrenergic agonists can be considered. We conclude that the use of SPS should be closely monitored for adverse GI complications, and the support of the gastroenterology team is mandatory when abdominal pain and constipation occur as in our patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Intestinal necrosis due to sodium polystyrene sulfonate-(Kayexalate) in sorbitol. South Med J. 2009;102:493-7.
- [Google Scholar]
- Sodium polystyrene sulfonate induced intestinal necrosis;A case report. Saudi Pharm J. 2018;26:771-4.
- [Google Scholar]
- Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J-Am Soc Nephrol. 1998;9:1924-30.
- [Google Scholar]
- Small bowel necrosis and perforation due to sodium polystyrene sulfonate in the setting of graft versus host disease and fulminant Clostridium difficile infection. J-Surg Case Rep. 2020;2020:rjaa253.
- [Google Scholar]
- Gastrointestinal adverse events with sodium polystyrene sulfonate-(Kayexalate) use:A-systematic review. Am J Med. 2013;126:264. e9-24
- [Google Scholar]







