Translate this page into:
Delayed Hematological Remission Predicts Poor Renal Outcome in Children with Atypical Hemolytic Uremic Syndrome
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
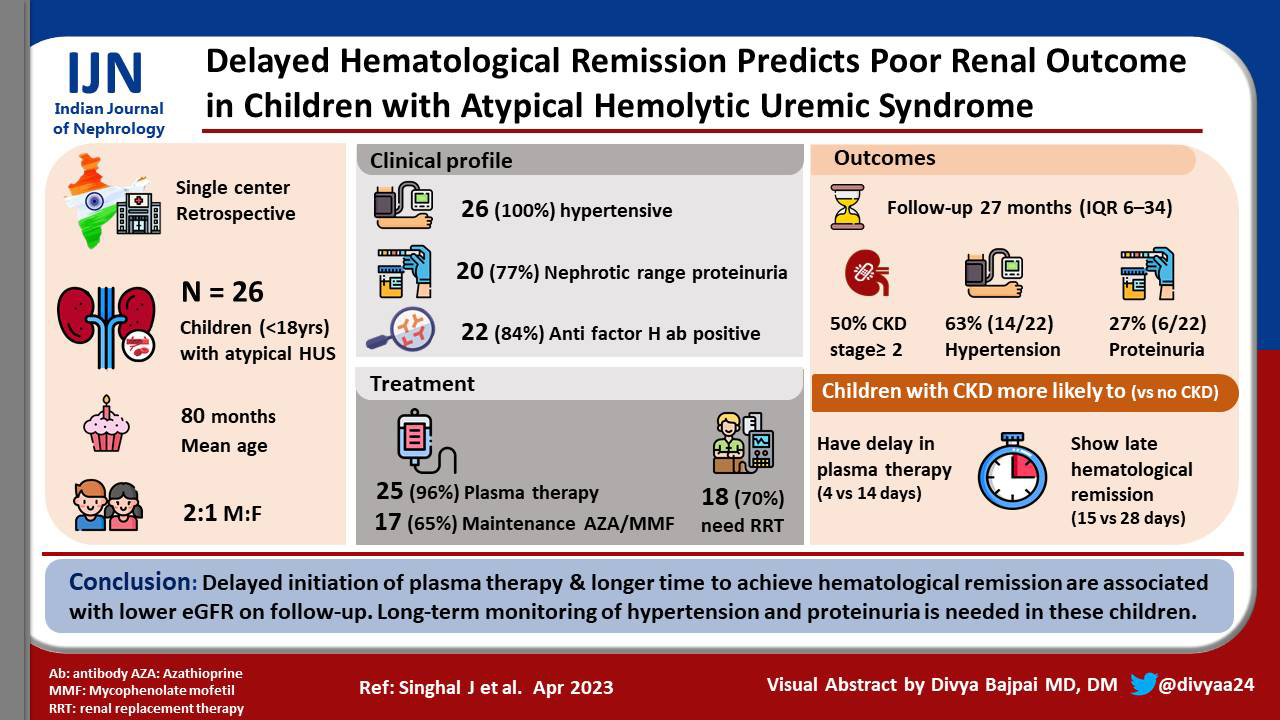
Background:
Atypical hemolytic uremic syndrome (aHUS) is hemolytic uremic syndrome (HUS) without a coexisting disease or specific infection. Eculizumab is the standard of care for children with aHUS. However, since it is not yet available in India, plasma therapy remains the treatment of choice in these patients. We studied the clinical profile of children with aHUS and the determinants associated with low estimated glomerular filtration rate (eGFR) on follow-up.
Materials and Methods:
A retrospective chart review of children (1–18 years) with aHUS managed at a tertiary care center was done. Demographic details, clinical features, and investigations at presentation and on subsequent visits were noted. Details of treatment and duration of hospital stay were recorded.
Results:
Of 26 children, boys outnumbered girls (2:1). The mean age at presentation was 80 ± 37.6 months. All children were hypertensive during the early phase of illness. Anti-factor H antibodies were elevated in 84% (22/26). Plasma therapy was initiated for 25 patients, and in 17 children, additionally immunosuppression was given. The median duration to achieve hematological remission was 17 days. As compared to children with normal eGFR, those with CKD stage 2 or more had significant delay in initiation of plasma therapy (4 vs. 14 days) and also took a longer time to achieve hematological remission (15 vs. 28 days). The prevalence of hypertension and proteinuria at the last follow-up was 63% and 27%, respectively.
Conclusion:
Delayed initiation of plasma therapy and longer time to achieve hematological remission are associated with lower eGFR on follow-up. Long-term monitoring of hypertension and proteinuria is needed in these children.
Keywords
aHUS
acute kidney injury
anti-factor H antibody
hypertension
plasma therapy
proteinuria
Introduction
Hemolytic uremic syndrome (HUS) is an important cause of acute kidney injury (AKI) in children. It is defined by the presence of microangiopathic hemolytic anemia (MAHA) (hemoglobin <10 g/dl, schistocytes ≥2% on peripheral smear, with either elevated lactate dehydrogenase [LDH] >450 IU/l or undetectable haptoglobin), thrombocytopenia (platelet count <150,000/mm3), and AKI. Patients are diagnosed to have atypical hemolytic uremic syndrome (aHUS) when there is no coexisting disease or specific infection identified. aHUS occurs mostly due to either abnormalities of the complement system or due to antibodies to complement factor H (CFH).[1] Overactivity of the alternative pathway can also occur because of gain-of-function mutations of factor B or C3 leading to aHUS. CFH is the most important protein for regulation of the alternate complement pathway, and presence of antibodies against this protein allows uninhibited activity of the complement cascade to occur on the surface of endothelial cells. Atypical HUS secondary to antibodies to CFH constitutes the majority of pediatric aHUS cases reported from India, while CFH mutation was responsible for 24%–27% aHUS cases in USA, Italy, and France.[2-5] Though eculizumab is the drug of choice for aHUS associated with inherited complement deficiencies and anti-factor H antibody–associated HUS, prompt treatment with plasma exchange (PLEX) combined with immunosuppression in the latter has been shown to have a favourable outcome in children when eculizumab is not available.[2,6]
There are very few reports from India regarding children with aHUS. We performed this study to record the clinical features and investigate the factors associated with short-term unfavourable outcomes in children with aHUS at a tertiary care center in India.
Materials and Methods
We extracted data from the case record forms of children with aHUS managed at our center from November 2013 to December 2019 with at least 3 months’ follow-up after discharge from the hospital. We excluded infants and patients who had received the initial treatment after diagnosis at another health facility. We noted the demographic details, presenting features, course in hospital, and details on follow-up after discharge. Laboratory investigations noted at presentation and at the last visit included complete hemogram, blood urea, serum creatinine, LDH, electrolytes, complement level C3, and anti-CFH antibody levels, urinalysis, and urine protein to creatinine ratio (uPCr). Renal biopsy and genetic study reports were noted where available.
Details of treatment with respect to number of plasma infusions (PI), PLEX sessions, use of immunosuppressive agents, and requirement of renal replacement therapy (peritoneal dialysis [PD], hemodialysis [HD]) were noted. The protocol for treatment of aHUS included daily PLEX with 60–75 ml/kg fresh frozen plasma (FFP) for 5–7 days, followed by three sessions per week for 1–2 weeks depending upon the course. Plasma exchange @ 15 ml/kg were instituted for children who were non-oliguric and in whom obtaining a vascular access for catheter insertion was difficult. The infusions were given daily till hematological remission and on alternate days thereafter up to attainment of renal recovery. After the guidelines for management of children with aHUS were published, cyclophosphamide/rituximab infusion and maintenance immunosuppression with corticosteroids, mycophenolate mofetil (MMF), or azathioprine were added to the management of our patients with positive anti-CFH antibodies.[6] The maintenance therapy was continued for a duration of 24 months while monitoring clinical, hematological, and biochemical renal profile. Following discharge from the hospital, these children were called for review every 1–2 months for the first year and every 2–3 months thereafter.
Hypertension was defined as blood pressure (BP) higher than 95th percentile for age, gender, and height.[7] Standard definitions were used for defining nephrotic range proteinuria. Hematological remission was defined as platelets >150,000/μl, schistocytes <2%, and LDH less than the upper limit of normal on two consecutive days. Relapse was defined as recurrence of anemia with schistocytes ≥2%, elevated LDH, and/or thrombocytopenia (platelets <150,000/μl) with or without AKI, following remission for >2 weeks. Children with normal estimated glomerular filtration rate (eGFR) on the last follow-up visit were compared to those with chronic kidney disease (CKD) stage 2 or more.
Statistical analysis
Continuous variables have been expressed as mean with standard deviation (SD) or median with interquartile range (IQR) as applicable. Differences in mean and median between groups were analyzed with unpaired t test and Mann–Whitney U test, respectively. Categorical data has been expressed as proportions, and the difference between two groups was analyzed using the Chi-square test. A P value < 0.05 was considered significant.
We obtained ethics clearance from the ethics committee of our institution. We have adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines in reporting this study.[8]
Results
Thirty-four children with aHUS were managed during the study period. After excluding three infants and five children who were managed elsewhere, 26 children formed our study cohort.
Baseline data
The demographic data, clinical features, and baseline laboratory parameters of the study participants are presented in Table 1. Apart from the common presentation described in the table, one girl presented with features of left ventricular failure and had severe systolic dysfunction on echocardiography. All children were hypertensive during the acute phase of illness, requiring an average of four antihypertensive agents for BP control. Three children (11.5%) had a normal platelet count on presentation, and renal biopsy prompted the diagnosis of HUS. In only one of these patients, platelet count decreased to 97,000/mm3 during the course of illness. Twenty-four patients had proteinuria at presentation, and it was in the nephrotic range transiently in 20 of them. Twelve children had gross hematuria, and six had microscopic hematuria. Low complement C3 levels were seen in 19 of 22 (86%) patients in whom it was tested. Anti-factor H antibody titres were high in 21 of the 22 (95%) patients who were tested. Histopathologic examination of kidney tissue was available in nine children; two showed chronic changes of thrombotic microangiopathy (TMA) and seven showed active glomerular or arteriolar TMA. Multiplex ligation probe amplification (MLPA) studies were available in five children; four showed a deletion in complement factor H–related (CFHR) 1 and 3 proteins and they all had anti-CFH antibody–associated HUS. Next-generation sequencing (NGS) in one child showed a mutation in CFH gene.
| Parameter | Mean±SD |
|---|---|
| Mean age at presentation in months (±SD) | 80±37.6 |
| Male: female | 2:1 |
| Clinical features | Vomiting (57.6%) |
| Gross hematuria and edema (46%) | |
| Oliguria (34%) | |
| Icterus (15.3%) | |
| Hemoglobin | 6.1±1.5 g/dl |
| Platelet count | 72,000±10,600/mm3 |
| Serum LDH | 2436±1331 IU/l |
| Serum creatinine | 4.4±3.44 mg/dl |
| eGFR | 17.4±11.4 ml/min/1.73 m2 |
aHUS=atypical hemolytic uremic syndrome, eGFR=estimated glomerular filtration rate, LDH=lactate dehydrogenase, SD=standard deviation
Treatment
Plasma therapy was initiated for 25 patients; four children received FFP infusions daily till hematological remission and later on alternate days (mean: 24 ± 3.5 sessions). Twenty-one children underwent a mean of 16 ± 9 PLEX sessions; 14 of them also received an average of 10 PI following exchanges. The median time to initiation of plasma therapy was 6 days (IQR: 3–9). Eighteen children needed renal replacement therapy (RRT) at presentation: HD in 14 children, PD followed by HD in three, and PD alone in one. The average duration of RRT was 14 days; one child progressed to CKD 5 and was withdrawn from further care. Intravenous cyclophosphamide was given to six patients, rituximab to two as induction therapy, and corticosteroids to all 19 patients with anti-CFH antibodies; 17 of them also received either azathioprine or MMF as maintenance therapy.
The median duration to achieve hematological remission from the start of plasma therapy was 17 days (IQR: 14–25), and the mean duration of hospital stay was 35 ± 18.8 days.
Follow-up
Relapses were seen in four patients (16%), all of whom had anti-CFH antibodies. The time to relapse was variable: within a month of discharge (one patient), 2 years later (two patients), and after 3 years of remission (one patient). Following discharge, details were available for 22 patients as one patient succumbed to the illness, two patients did not return, and one boy initially presented like a steroid-resistant nephrotic syndrome (SRNS) without hematological features and it was the renal biopsy that confirmed the diagnosis of HUS. His proteinuria was controlled with an angiotensin converting enzyme (ACE) inhibitor. He remained well for 2 years, following which he presented with the classic triad of HUS and had high anti-CFH antibody levels.
The median duration of follow-up was 27 months (IQR: 6–34 months). Fifty percent had CKD stage 2 or more (stage 2 in all except one who was dialysis dependent). Hypertension persisted in 63% (14/22) and proteinuria in 27% (6/22). We compared children with normal eGFR to those who had CKD 2 or more. Though they were comparable in mean age, mean eGFR at presentation, proportion requiring RRT, and mean length of hospital stay, those with >CKD 2 had a lower nadir eGFR during therapy, longer time to initiation of plasma therapy, and longer time to achieve hematological remission [Table 2].
| Parameter | eGFR≥90 ml/min/1.73 m2 (n=11) | eGFR<90 ml/min/1.73 m2 (n=11) | P |
|---|---|---|---|
| Mean age at presentation (months) | 83.3±21.2 | 86.64±17.6 | 0.42 |
| Mean duration of follow-up (months) | 23.6±18 | 29.7±15 | 0.23 |
| Mean eGFR at presentation (ml/min/1.73 m2) | 18.53±11.6 | 13.5±3.8 | 0.09 |
| Mean lowest eGFR during illness (ml/min/1.73 m2) | 18.34±1.6 | 9.85±4.3 | 0.03 |
| Mean eGFR at the last follow-up (ml/min/1.73 m2) | 95.5±4.2 | 74.95±53 | 0.03 |
| Mean time to hematological remission (days) | 15.1±4.2 | 28±19.7 | 0.017 |
| Need of dialysis | 54.5% | 90% | 0.05 |
| Mean number of days between the onset of symptoms and initiation of plasma therapy (days) | 4.09 | 14.73 | 0.02 |
| Length of hospital stay (days) | 29±14.8 | 40±1.4 | 0.05 |
| Average no. of antihypertensives | 1.64 | 3.55 | 0.03 |
| Proteinuria (%) | 27% | 36.3% | 0.64 |
| Hypertension (%) | 54.5% | 81.8% | 0.16 |
eGFR=estimated glomerular filtration rate
Discussion
We describe the clinical profile of 26 children with aHUS who were managed with plasma therapy, immunosuppression when anti-factor H antibodies were present, and RRT when indicated. We compared the clinical and laboratory parameters of those whose kidney function returned to normal to those whose eGFR remained low.
A diagnosis of aHUS is reserved for patients with HUS without a coexisting disease or specific infection. It occurs due to overactivation of the alternative complement pathway due to either hereditary mutations in complement genes or autoantibodies against CFH.[9] The presence of autoantibodies directed against CFH resulting in its functional deficiency was first published in 2005.[10] The mean age of presentation in our study cohort was 6.8 ± 1.9 years, majority of whom were anti-CFH antibody positive. Sinha et al.[2] reported data on children with aHUS from multiple centers in India. The mean age of presentation was 8.4 ± 4.1 years in those with anti-CFH antibody–associated aHUS and 5.1 ± 4.9 years in those without the antibodies. Fremeaux-Bacchi et al.[4] described 89 children with aHUS; of these, 56% who had inherited defects of complement experienced disease onset before 2 years of age, while only 11% cases were due to anti-CFH antibodies and developed symptoms between 2 and 12 years of age. Dragon Durey et al.[11] reported that the median age of presentation was 8.5 years and male to female ratio was 2.5:1 in children with anti-CFH antibody–associated aHUS, similar to our finding.
In a small proportion of patients, the complete triad of HUS may be absent at presentation and manifests only during the course of illness. Three of our patients did not have thrombocytopenia at onset. One of them presented with features of chronic glomerulonephritis with intermittent episodes of gross hematuria and periorbital puffiness for 1 month. Another behaved like an SRNS and the third presented with features of rapidly progressing glomerulonephritis (gross hematuria, edema with serum creatinine of 10.5 mg/dl). All three had histopathologic features of microangiopathy on renal biopsy. Approximately 15% children may even have a normal serum creatinine at presentation.[12] The mean serum creatinine at presentation in our cohort was 4.4 ± 3.44 mg/dl, and 69% required RRT. Peritoneal Dialysis was performed in one child due to technical difficulty, and it was the initial modality in two other children who were hemodynamically unstable. Puraswani et al. similarly reported a mean serum creatinine of 5.56 ± 2.98 mg/dl, and 77.3% of children needed some form of RRT for a mean duration of 15 days.[13] The requirement of RRT has been variously reported to be 44%–95% in children with aHUS.[4,10,14,15]
Arterial hypertension is frequent and often severe in patients with HUS due to both oliguria/anuria leading to volume overload and hyperreninemia secondary to renal TMA. The prevalence of hypertension at presentation has been reported to range from 54% to 88%.[5,11,13-17] All our patients were hypertensive at presentation or during the early phase of illness. Geerdink et al. found the overall prevalence of hypertension to be 71% in children with aHUS and 100% in those with positive anti-CFH antibodies.[14] We found 83% (20/24) of children to have nephrotic range proteinuria in contrast to 56.7% as noted in another Indian cohort.[13] Hematuria, either gross/microscopic, was present in 72% of children, similar to that reported by Brockelbank.[18]
The prevalence of anti-CFH antibody–associated HUS has been reported to be 4% and 7% in two studies evaluating both adults and children with aHUS, respectively.[5,10] Studies from outside India which evaluated only pediatric patients with aHUS found a varied prevalence ranging from 9% to 26%. Lee et al. studied a Korean pediatric cohort with aHUS and found 29% of children to be anti-CFH antibody positive.[19] The prevalence of anti-CFH antibody–associated HUS in our patients was 95%, while Puraswani et al. reported that among 781 Indian children with aHUS, 56% had anti-CFH antibodies.[13-15,18,20,21]
Low C3 levels have been reported in 30% of children with aHUS.[4,5,9,14,22] Hypocomplementemia was present in 86% of our patients at presentation, similar to that reported by Uthup et al. who found it to be 88% in children with “diarrhea-negative HUS,” though measurement of anti-CFH antibody levels or mutational analysis to detect defects in complement regulatory genes was not performed by them.[17] Sinha et al. reported that 62% of children with anti-CFH antibodies had low complement levels and there was a negative correlation between the two. In addition, levels of anti-factor H antibodies greater than 8000 AU/ml correlated with poor outcome.[1] We did not attempt to find any correlation between anti-CFH antibody titres and renal outcomes as the assay for antibody was restandardized during the study period (upper limit of normal 100 AU/ml in 2017 and 20 AU/ml before that).
Renal biopsy is indicated in children with aHUS in case of unclear diagnosis or unsatisfactory clinical response to plasma therapy.[23] The former was the reason for biopsy in three of our patients who had normal platelet counts at presentation. Six children underwent a biopsy because of inadequate response to therapy. Three demonstrated features of chronic TMA and six revealed acute activity, and hence, treatment was continued.
Genetic evaluation is not required for urgent therapeutic decisions, but is necessary to establish the etiology, to predict the risk of relapses and risk of progression to end-stage kidney disease (ESKD), for genetic counseling, and for making decisions regarding duration of complement blockade treatment when available and prior to kidney transplantation. The strong correlation between the occurrence of CFH autoantibodies and absence or reduction of CFHR1/CFHR3 in plasma was first reported by Joszi et al.[20] Multiplex ligation-dependent probe amplification in five of our patients with positive CFH antibodies revealed homozygous deletion in both CFHR1 and CFHR3 in four patients. One patient who was CFH antibody positive had a homozygous deletion in CFH gene on NGS. In cohorts reported by Hofer et al., Jozsi et al., and Moore et al., homozygous deletion of CFHR1 had been noted in 80% patients.[15,20,21] As we could not perform MLPA for all our anti-CFH antibody–associated aHUS patients, this does not reflect the true prevalence of genetic mutations in our cohort. Mutations in other complement genes like CFH, CFI, CD46, and C3 variants have been reported in addition to presence of anti-CFH antibodies, thereby suggesting multiple susceptibility factors in some patients to manifest the disease.[21] In addition, up to 12% of patients with aHUS have been found to have various combinations of two or more mutations of CFH, CFI, MCP, C3, CFB, or thrombomodulin.[3,5,22,24]
Eculizumab has been proposed as the first-line treatment, and if it is not available, it is recommended to initiate early PLEX or PI (if PLEX is not possible).[6] We initiated plasma therapy in 25 patients within a median of 6 days from the onset of symptoms (IQR: 3–9 days). The delay was caused because these patients were referred to us after initial evaluation and symptomatic treatment at centers of primary care. Puraswani et al. reported patients from different centers from India; median time to initiation of therapy was 12 days (IQR: 6–24).[13] Sinha et al. showed that delayed initiation of PLEX, 2–3 weeks following onset, predicted adverse outcomes.[2] We too found that children who had CKD stage 2 or more had delayed initiation (4 vs. 14 days) of plasma therapy compared to the group with normal eGFR. The time to achieve hematological remission in our patients was a median of 17 days in contrast to 27 days reported by Puraswani et al.[13]
A relapse proportion of 40%–50% has been reported in children with aHUS.[4,14] Of those with anti-CFH antibodies, 11%–43% showed relapses, with majority showing them in the first 2 years after the first episode.[2,11,13,18,21] Dragon-Durey et al.[10] summarized treatment outcomes in 243 patients with anti-CFH antibody–associated HUS and found that 10.7% patients treated with PLEX and immunosuppression relapsed, compared to 28.7% of those treated with PLEX alone in previously published studies. Sixteen percent (4/26) of our patients relapsed. All four had anti-CHF antibodies; two of these children had been treated with PLEX and steroids, one received PLEX alone (managed before the publication of the guidelines), and one had presented as SRNS (described above). The relapses were treated with PLEX and immunosuppression.
Twenty-two children were followed up for a median of 27 months. An equal number had CKD stage 2 or more and eGFR >90 ml/min/1.73 m2 [Table 2]. The mean age at presentation and median duration of follow-up were similar in these two groups, but the mean eGFR at the last visit was significantly different, justifying our objective of comparing these two groups with respect to factors contributing to lower residual eGFR. The time to initiate plasma therapy and the mean number of days to achieve hematological remission were significantly higher in children with CKD stage 2 or more. More children in the latter group needed RRT during the acute illness, but the difference was not statistically significant. Delayed hematological remission and need for prolonged dialysis were associated with adverse outcome, defined as CKD stage 4/5 or death in children with anti-CFH antibody–associated HUS.[2] Anti-CFH antibody–associated aHUS has been associated with a high rate of ESKD (27%–63%).[2,5,10,25] The low proportion of ESKD (5.2%) in our patients is explained by the short duration of follow-up.
Geerdink et al.[14] found chronic hypertension in 54% of their patients with aHUS and 47% had sustained proteinuria. Puraswani et al. reported prevalence of stage 2 hypertension or proteinuria in 42.7% of children with anti-CFH antibody–associated HUS at 3 months of follow-up.[13] The prevalence of hypertension and proteinuria in our cohort was 68% and 31.8%, respectively.
The mortality in our patients was 7.6% (median follow-up: 27 months); one child presented late and the other was withdrawn from treatment by caretakers. The French cohort included 89 children with 11% anti-CFH–associated disease; mortality was 7.8% during a median follow-up period of 45 months.[4] Geerdink et al.[14] managed 45 children with aHUS with plasma therapy (13% anti-CFH antibody associated) and reported a mortality of 9% during a follow-up period of 7.5 years. Puraswani et al.[13] recorded a mortality of 6.5% in children with anti-CHF–associated HUS managed with plasma therapy and immunosuppression. The high mortality in our patients could be attributed to the delay in reaching our center and noncompliance with therapy.
The limitation of our study is its retrospective observational design and small sample size. Since new literature on management of aHUS was emerging, there was heterogeneity in treatment of our patients. Secondly, we did not look for other genetic defects in those in whom we had found one (e.g., we did not perform NGS in those where a copy number variation had been identified by MLPA).
Conclusion
This single-center study showed better outcome in children with anti-factor H antibodies when they were given early therapy with PLEX and immunosuppression, with lower morbidity in terms of developing chronic kidney disease stage 2 or more. Delayed hematological remission identifies those likely to progress to CKD 2 or more. Children with aHUS have persistent hypertension and proteinuria despite attaining hematological remission and irrespective of their CKD stage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal Involvement in Children with HUS. In: Avner, ed. Pediatric Nephrology (7th ed). Berlin Heidelberg: Springer-Verlag; 2016. p. :1490-513.
- [Google Scholar]
- Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 2014;85:1151-60.
- [Google Scholar]
- Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010;31:1445-60.
- [Google Scholar]
- Genetics and outcome of atypical hemolytic uremic syndrome:A Nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554-62.
- [Google Scholar]
- Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844-59.
- [Google Scholar]
- An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15-39.
- [Google Scholar]
- Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904.
- [Google Scholar]
- The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement:Guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-7.
- [Google Scholar]
- Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555-63.
- [Google Scholar]
- Clinical features of anti-factor H autoantibody–associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21:2180-7.
- [Google Scholar]
- Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18:2392-400.
- [Google Scholar]
- Clinical and immunological profile of anti-factor H antibody associated atypical hemolytic uremic syndrome:A nationwide database. Front Immunol. 2019;10:1282.
- [Google Scholar]
- Atypical hemolytic uremic syndrome in children:Complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27:1283-91.
- [Google Scholar]
- Complement factor H-antibody–associated hemolytic uremic syndrome:Pathogenesis, clinical presentation, and treatment. Semin Thromb Hemost. 2014;40:431-43.
- [Google Scholar]
- Characteristics and outcome of hemolytic uremic syndrome in Sudanese children in a single Centre in Khartoum State. Sudan J Paediatr. 2017;17:42-8.
- [Google Scholar]
- Clinical profile and outcome of D- haemolytic-uraemic syndrome in children from south India. South Afr J Child Health. 2014;8:68.
- [Google Scholar]
- Factor H autoantibody is associated with atypical hemolytic uremic syndrome in children in the United Kingdom and Ireland. Kidney Int. 2017;92:1261-71.
- [Google Scholar]
- Atypical hemolytic uremic syndrome:Korean pediatric series:Korean pediatric aHUS cohort. Pediatr Int. 2015;57:431-8.
- [Google Scholar]
- Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood. 2008;111:1512-4.
- [Google Scholar]
- Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood. 2010;115:379-87.
- [Google Scholar]
- Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2008;23:1957-72.
- [Google Scholar]
- Hemolytic uremic syndrome in a developing country:Consensus guidelines. Pediatr Nephrol. 2019;34:1465-82.
- [Google Scholar]
- Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 2010;77:339-49.
- [Google Scholar]
- Characterization of complement factor H–related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome Blood. . 2009;114:4261-71.
- [Google Scholar]







