Translate this page into:
Double ANCA-positive vasculitis in a patient with infective endocarditis
Address for correspondence: Dr. Ilangovan Veerappan, Department of Nephrology, Pondicherry Institute of Medical Sciences, Ganapathichettikulam, Village No. 20, Kalapet, Puducherry - 605 014, India. E-mail: ilangovanv@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The most common pattern of renal involvement in infective endocarditis is infection-associated glomerulonephritis. Due to clinical symptoms and signs that overlap with vasculitis, the diagnosis of infective endocarditis may be delayed. The unusual combination of reduced complement with positive antineutrophil cytoplasmic antibody should raise the suspicion of infections such as infective endocarditis.
Keywords
Antineutrophil cytoplasmic antibody
endocarditis
vasculitis
Introduction
Infectious diseases, especially those that can mimic vasculitis, are of particular importance in the differential diagnosis of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) because the misdiagnosis of an infectious disease as AAV, and the administration of immunosuppressive therapy could promote the infection and produce disastrous consequences. Thus, an accurate understanding of ANCA test specificity in infectious diseases is critical. A variety of infectious diseases have been reported to be associated with ANCA. The implication of the presence of ANCA in infectious diseases remains unclear. This case suggests that somehow the infectious process induces the production of ANCA, possibly through non-specific B-cell activation or auto-immunization after the release of proteinase 3 (PR3) from neutrophils. The ANCA may contribute to the inflammatory process. When encountered with ANCA positivity in patients suspected of having systemic vasculitis, physicians should take appropriate steps to rule out infectious diseases, including sub-acute bacterial endocarditis (SBE), before committing to long-term immunosuppressive therapy.
Case Report
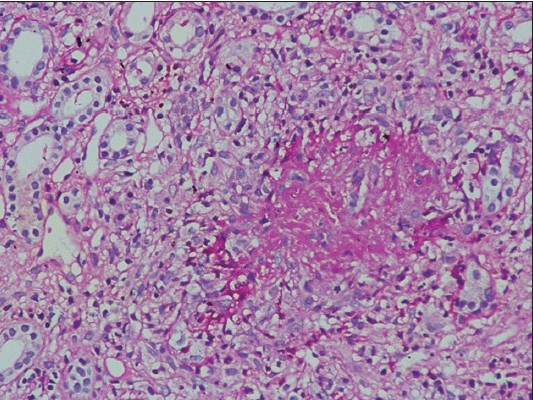
A 45-year-old male consulted a general practitioner for his pedal edema of 1 week duration. He was detected to have renal dysfunction and was referred to us. The patient was unwell, 2 months after a tooth extraction and had consulted three doctors for his clubbing and generalized feeling of lassitude. He never had any medical illness in the past and was told to be normal after evaluation. The details of those evaluations were not available. The patients also had dyspnea on doing more than ordinary work for 6 months and had paroxysmal nocturnal dyspnea (PND) of 1 month duration. The clinical examination showed pallor, pan-digital clubbing and pedal edema. The blood pressure was 110/70 mm Hg, pulse rate 98/min, and respiratory rate 26/min. The kidneys were 9.5 cm long on both sides with increased cortical echogenicity, hemoglobin was 6.5 g/dl, serum urea 107 mg/dl, serum creatinine 4.5 mg/dl, Modified Diet in Renal Disease estimated glomerular filtration rate (MDRD e-GFR) 23 ml/min/1.73 m2), and normal WBC count (total count 5600/mm3). The urine showed microscopic hematuria (RBC > 100/hpf), hyaline, granular casts, and 24-h proteinuria was 650 mg/day. The chest X-ray film showed normal findings. The dyspnea and PND improved with blood transfusion and diuretics. The renal biopsy showed pauci-immune fibro-cellular crescentic vasculitis [Figures 1a and b] with vessel necrosis and inflammation [Figure 1c].

- Renal biopsy showing cellular crescent around a glomerulus (H and E, 400×10)
- Renal biopsy showing interstitial granuloma with interstitial inflammation with loss of tubular architecture (H and E, 400×10)
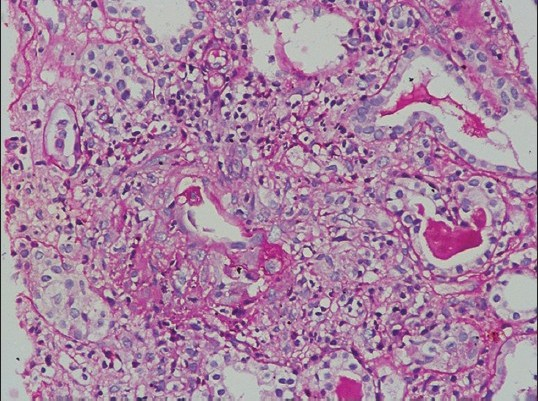
- Renal biopsy showing vascular inflammation and areas of necrosis (H and E, 400×10)
The c-ANCA and p-ANCA were elevated, and the C3 complement was repeatedly low and C4 was low normal. Three doses of 0.5 g of injectable methylprednisolone were given followed by oral steroid (0.5 mg/kg/day) along with oral cyclophosphamide (1 mg/kg/day). The renal functions improved and the serum creatinine stabilized at 1.8 mg/dl. Echocardiography showed small vegetations in the mitral leaflets with severe mitral regurgitation, moderate severe mitral stenosis, and severe pulmonary arterial hypertension. The patient was characterized as “possible infective endocarditis” as per the modified Duke's criteria for infective endocarditis with one major criteria – positive echocardiogram for infective endocarditis with an oscillating intra-cardiac mass on the mitral valve, and two minor criteria: (1) predisposing rheumatic mitral valve disease with a recent history of tooth extraction and (2) immunological renal involvement in the form of dual ANCA-positive vasculitis. The cyclophosphamide was stopped and steroids were rapidly tapered and stopped. The vegetations grew in size over the next 4 weeks despite empirical antibiotics for culture-negative endocarditis. Due to absence of history of fever or leukocytosis and as all the blood cultures were negative, it was not clear if the patient had infective endocarditis or the small vegetations were secondary to vasculitis. The vegetation continued to grow despite vancomycin, cefaperazone/sulbactum therapy, and hence the valve was replaced with a bioprosthetic valve. The abnormal valve with the vegetation is shown in Figure 2. The C3 normalized within a month after valve replacement, c-ANCA was negative after 2 months, and p-ANCA normalized when it was tested after 4 months. The immunosuppression was stopped within 2 weeks after diagnosis and the patient continues to be stable over the 4 months follow-up now.
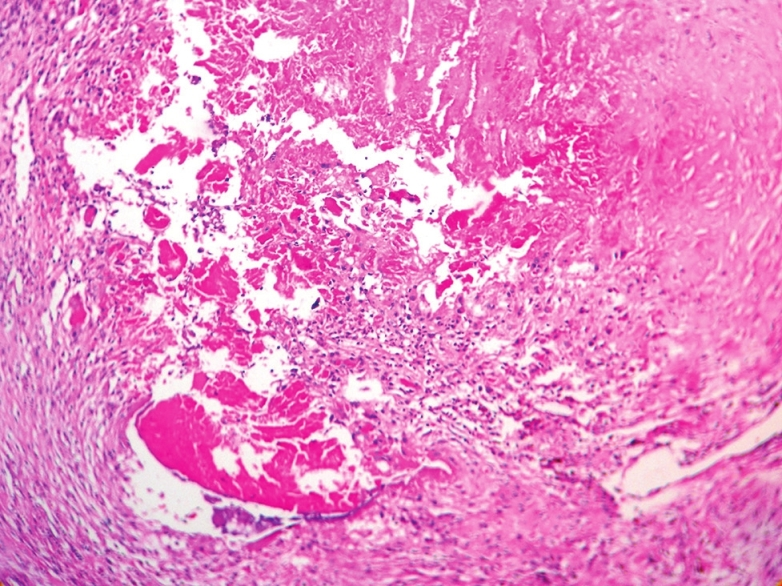
- Histology of the valve with infective endocarditis. The figure shows a vegetation attached to the valve leaflet
Discussion
Cardiac involvement is rare in ANCA-associated vasculitis.[1] Both ANCA-associated small vessel vasculitis (SVV) with endocardial compromise and SBE have overlapping clinical manifestations such as valvular vegetations, inflammatory signs, renal involvement, and constitutional symptoms. Aortic valve is almost always involved in ANCA-associated SVV and is associated with skin, renal, respiratory involvement, and normal complement levels with no splenomegaly.[2]
A variety of infectious and non-infectious diseases could sometimes result in false-positive ANCA tests by indirect immunoflorescence (IF). However, they are associated with negative specific Enzyme Linked Immunosorbent Assay (ELISA) testing for anti-PR3 or anti-MPO.[3] In our case, the c-ANCA and p-ANCA were positive by IF and ELISA testing which confirmed that they were specific for anti-PR3 and anti-MPO.
The initial vegetations were small and discrete raising the suspicion of vasculitis-induced cardiac involvement. The patient's symptoms started months after tooth extraction, and the pattern of valve involvement was suggestive of rheumatic heart disease. Cardiac involvement in WG is more common than generally thought, ranging from 6% to 44% of patients[1] and includes coronary arteritis, pericarditis, myocarditis, valvulitis/endocarditis, conduction system granulomata, sinus node arteritis, AV node arteritis, myocardial infarction, and epicarditis.[4]
In addition to infections, ANCA has been reported in heroin users in the absence of HCV or other infections.[5] Antithyroid drug-induced ANCA-positive vasculitis is usually p-ANCA positive with high titers and skin is involved in nearly two-third of patients. Other organs are rarely involved.[6]
Bacterial endocarditis can also be associated with ANCA antibodies,[7–9] rarely combined c-ANCA and p-ANCA positivity.[1011] Infections have been considered to be one of the potential triggering factors for ANCA-associated vasculitides.[12] Bacterial endocarditis can mimic clinical vasculitis[13] and should be in the differential diagnosis of idiopathic systemic necrotizing vasculitides.
Both idiopathic and AAV associated with infections cannot be discriminated by the pattern of renal and skin lesions. However, AAV patients more frequently had pulmonary and nervous system manifestations. Patients with infections more frequently had dual ANCA (high PR3, low MPO), anti-cardio lipid antibodies, anti-beta2-GP I, cryoglobulins, and hypocomplementemia.[14]
As seen in our case there can be discrepancy between clinical recovery and the titers of ANCA.[15] ANCA levels fall or become negative in 30-80% of patients within the first 1-3 months of induction treatment in primary AAV. In AAV, persistence of ANCA beyond this point is usually associated with ongoing disease activity, but most patients ultimately reach remission and become ANCA-negative.[16] But little is known about the time to recovery of the ANCA titers after treatment for infective endocarditis in patients with infective endocarditis-associated ANCA vasculitis. In our case, the c-ANCA had normalized with a month after valve replacement, but the p-ANCA normalized only around 4 months.
Conclusion
Double-positive ANCA with low serum complements is seen in SBE-associated vasculitis. Early diagnosis avoids complication of immunosuppression in a patient with infective endocarditis. Complements and c-ANCA rapidly normalize upon recovery from infective endocarditis. There is poor correlation between p-ANCA titers and recovery from infective endocarditis and infection-associated vasculitis.
Source of Support: Nil
Conflict of Interest: None declared
References
- Endocarditis associated with antineutrophil cytoplasmic antibodies: A case report and review of the literature. Clin Rheumatol. 2007;26:590-5.
- [Google Scholar]
- ELISA is the superior method for detecting antineutrophil cytoplasmic antibodies in the diagnosis of systemic necrotising vasculitis. J Clin Pathol. 1999;52:670-6.
- [Google Scholar]
- Cardiac complications of Wegener granulomatosis: A case report of complete heart block and review of the literature. Semin Arthritis Rheum. 1980;10:148-54.
- [Google Scholar]
- Clinical significance of antinuclear antibodies, anti-neutrophil cytoplasmic antibodies and anticardiolipin antibodies in heroin abusers. Isr Med Assoc J. 2002;4:908-10.
- [Google Scholar]
- Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: Comparison with idiopathic ANCA vasculitides. Arthritis Res Ther. 2005;7:R1072-81.
- [Google Scholar]
- Infection with the human immunodeficiency virus type 1 and vascular inflammatory disease. Clin Exp Rheumatol. 2004;226:S87-93.
- [Google Scholar]
- Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis Rheum. 2000;43:226-31.
- [Google Scholar]
- Microscopic polyangiitis following recurrent Staphylococcus aureus bacteremia and infectious endocarditis. Clin Exp Rheumatol. 2006;24:705-6.
- [Google Scholar]
- High prevalence of antineutrophil cytoplasmic antibody positivity in childhood onset Graves’ disease treated with propylthiouracil. J Clin Endocrinol Metab. 2000;85:4270-3.
- [Google Scholar]
- Bacterial infection presenting as cutaneous vasculitis in adults. Clin Exp Rheumatol. 1999;17:471-3.
- [Google Scholar]
- Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: Diagnostic and therapeutic challenge. Clin Rheumatol. 2010;29:893-904.
- [Google Scholar]
- Crescentic glomerulonephritis associated with infective endocarditis: Renal recovery after immediate surgical intervention. Clin Exp Nephrol. 2000;4:329-34.
- [Google Scholar]
- The current status of neutrophil cytoplasmic antibodies. Clin Exp Immunol. 1989;78:143-8.
- [Google Scholar]







