Translate this page into:
Double Anti-neutrophil Cytoplasmic Antibody and Anti-glomerular Basement Membrane Antibody-positive Crescentic Glomerulonephritis, Following SARS-CoV-2 Infection
Address for correspondence: Dr. Rajeev A. Annigeri, Department of Nephrology, 21, Gream's Lane, Chennai - 600 006, Tamil Nadu, India. E-mail: rajeevnephro@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affects kidneys mainly in the form of acute kidney injury but rarely can cause glomerular disease. On a very rare occasion, SARS-CoV-2 infection can be associated with anti-neutrophil cytoplasmic antigen (ANCA)-associated vasculitis and anti-glomerular basement membrane glomerulonephritis (anti-GBM GN). We report a case of a 59-year-old man who presented with progressive renal failure 8 weeks after contracting the viral infection, which progressed slowly to severe renal dysfunction. Renal biopsy showed crescentic glomerulonephritis (CrGN) accompanied by interrupted linear IgG deposits along the glomerular basement membrane (GBM) on immunofluorescence (IF) staining with associated mild acute tubular injury. The serology for anti-myeloperoxidase (MPO), as well as anti-GBM antibodies, was positive. He was treated with steroid and pulse intravenous cyclophosphamide, following which there was a significant improvement in the renal function and serological resolution of both the antibodies 6 months post-treatment. To the best of our knowledge, this is the first reported case of “double-antibody” positive CrGN following SARS-CoV-2 infection.
Keywords
ANCA-associated vasculitis
anti-GBM disease
double-positive
SARS-CoV-2
Introduction
The novel virus SARS-CoV-2 predominantly affects the respiratory tract, but several other organs, including kidneys, may also be involved in some cases. Renal manifestation in SARS-CoV-2 infection is acute kidney injury (AKI) in most cases, which occurs in about 9% of hospitalized patients.[1] However, glomerular involvement with SARS-CoV-2 infection in the form of podocytopathy causing collapsing glomerulopathy, IgA nephropathy, membranous nephropathy, minimal change disease, thrombotic microangiopathy, anti-neutrophil cytoplasmic antigen (ANCA) associated vasculitis, and anti-glomerular basement membrane glomerulonephritis (anti-GBM GN) have also been reported.[2] Very few cases of ANCA-associated vasculitis (AAV)[3456] and anti-GBM GN have been reported thus far.[78910] In non-SARS-CoV-2-infected patients, the concurrent occurrence of AAV and anti-GBM GN is seen occasionally, but such a phenomenon has not been documented following the COVID infection.
We report a case of crescentic glomerulonephritis (CrGN) manifesting 2 months after SARS-CoV-2 infection, with “double-positive” ANCA and anti-GBM antibodies and CrGN with linear IgG immunofluorescence (IF) deposits along the glomerular basement membrane (GBM).
Case Presentation
A 59-year-old man, who was previously healthy, developed fever and cough and was diagnosed to have SARS-CoV-2 infection by nasal swab reverse transcriptase-polymerase chain reaction test in August 2020 elsewhere. The clinical course was mild and he did not have significant hypoxia and was hospitalized for 10 days at a Government hospital. He did not receive paracetamol and multivitamins and did not receive steroids, remdesivir, or any other medicines during hospitalization. Serum creatinine (SCr) was normal during hospitalization. He had well-controlled hypertension for 7 years. During the evaluation for malaise and tiredness, he was detected to have mild azotemia (SCr: 1.49 mg/dl) on October 6, 2020. Subsequently, he developed progressive weakness, and SCr was 3.5 mg/dl in November 2020; urine examination showed 2+ albumin and 3-5 RBCs/hpf. He came to our unit for further evaluation on December 19, 2020. On examination, he had mild pallor, blood pressure was 120/70 mm Hg, and there was no pedal edema or skin rash. The systemic examination was unremarkable. Laboratory results were as follows: urine albumin 1+, RBC 1-3/hpf, WBC 3-5/hpf, urine protein creatinine ratio 0.7 g/g creatinine, blood urea 90 mg/dl, SCr 4.9 mg/dl, serum sodium 117 meq/L, serum potassium 4.5 meq/L, serum chloride 90 meq/L, serum HCO3 21 meq/L, random blood sugar 119 mg/dl, serum protein 6.6 g/dl, serum albumin 3.9 g/dl, serum cholesterol 151 mg/dl, serum calcium 8.6 mg/dl, serum phosphorus 3.9 mg/dl, serum bilirubin 0.2 mg/dl, serum ALT 13 U/L, serum alkaline phosphatase 50 U/L, hemoglobin 8.9 g/dl, WBC count 8,010/mm3, platelet count 351,000/mm3, and serum immunotyping showed no monoclonal gammopathy. Serum Covid-19 IgG antibody was positive. Ultrasonography showed normal-sized kidneys with increased cortical echoes. The ANA and anti-ds DNA were negative, whereas p-ANCA was positive on IF. Serum anti-myeloperoxidase (MPO) antibody by ELISA was strongly positive (134.86 RU/ml, normal: <20 RU/ml), whereas anti-proteinase 3 (PR3) antibody was normal (3.27 RU/ml).
A kidney biopsy was done, and light microscopy revealed 33 glomeruli, of which 10 showed ischemic obsolescence. The viable glomeruli showed mesangial and endocapillary proliferation with some exudation [Figure 1a and b]. Partial and circumferential fibrous and fibroepithelial crescents were seen in 13 out of 33 glomeruli [Figure 1c and d]. Occasional synechiae formation was noted. Periglomerular granulomatous and inflammatory reaction was seen focally with rupture of the GBM [Figure 1e and f]. There was multifocal tubular atrophy and interstitial fibrosis (IFTA 20-30%). The blood vessels showed mild to moderate arteriosclerotic changes with some hyalinization. The IF study showed linear staining in IgG (2 to 3+) [Figure 2] interrupted by coarse granularity focally, peripheral, and mesangial granular C3c (3+) deposits. Though the morphology in this clinical setting suggested the AAV process, the presence of linear IgG staining along the GBM raised suspicion of concomitant anti-GBM GN. Serum anti-GBM antibody was positive (61.27 RU/ml, negative: <20 RU/ml).

- (a) Renal biopsy showing proliferating glomeruli with some of them showing crescent formation; PAS stain, 40×. (b) Glomerulus showing segmental proliferation with exudation and early crescent formation; PAS stain, 400×. (c and d) Fibrous and fibrocellular crescents; H and E and PAS stains, 400×. (e and f) Glomeruli with ruptured Bowman's capsule and severe periglomerular histiocytic reaction; PAS and H and E Stains, 400×
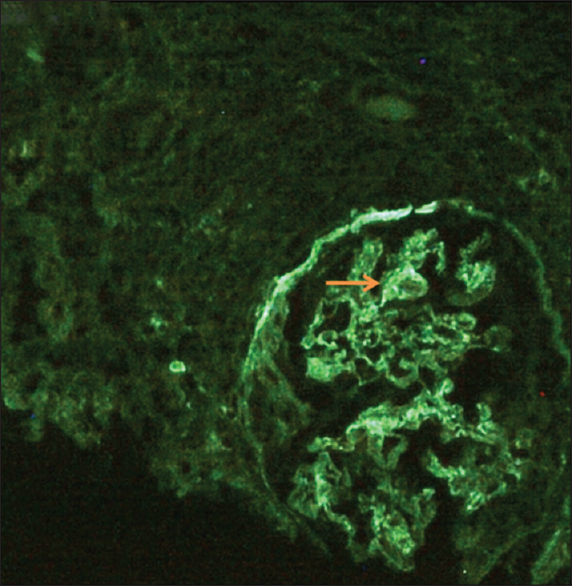
- Linear immunofluorescence on IgG (++) staining along the glomerular capillary walls interrupted by coarse granularity at foci (arrow)
He received intravenous methylprednisolone 250 mg once daily for 3 days followed by oral prednisolone 60 mg once daily, the dose of which was tapered to 30 mg by 4 weeks, 20 mg by 6 weeks, and 7.5 mg at 6 months. He also received pulse intravenous cyclophosphamide once a month for 5 months (cumulative dose: 3100 mg). He did not require dialysis and was advised plasma exchange for the removal of anti-GBM antibodies, which he declined. Serum p-ANCA by IF was positive while anti-GBM antibody by IF was negative after 3 weeks. Both ANCA and anti-GBM antibodies were negative by IF after 5 months. After 5 months, mycophenolate mofetil sodium 540 mg daily was introduced for maintenance immunosuppression. SCr showed a progressive decline and was 2.4 mg/dl when last seen on July 5, 2021. He is planned to continue low-dose steroids and MMF for maintenance immunosuppression.
Discussion
We report a case of CrGN with linear IgG deposits along the GBM and “double-positive” anti-MPO and anti-GBM antibodies in an elderly male following mild SARS-CoV-2 infection. Our patient had several unique features, which are mentioned as follows. 1) He presented with CrGN with double-positive autoantibodies and linear IgG immunofluorescence staining along the GBM. 2) He presented with predominant AAV phenotype rather than anti-GBM GN, whereas anti-GBM phenotype predominates in most double-positive cases.[11] 3) The renal disease appeared 8 weeks after the onset of SARS-CoV-2 infection indicating that inflammatory milieu and immune response probably had a role in developing autoimmunity. 4) The clinical presentation was predominant with constitutional symptoms with mild proteinuria and no hematuria; kidney biopsy showed predominantly fibroepithelial crescents, and the clinical course was indolent with relatively slow progression for a CrGN. 5) ANCA positivity lasted much longer, whereas anti-GBM antibodies disappeared from circulation within 3 weeks following immunosuppression and without plasma exchange therapy. 6) He responded favorably to immunosuppressive treatment with improvement in GFR as well as general health.
Prendecki et al.[7] reported an unusual regional increase in the incidence of anti-GBM disease in the east United Kingdom during the SARS-CoV-2 pandemic. They reported eight cases of anti-GBM GN during 5 months, four out of which were confirmed to have previous infections with SARS-CoV-2. Several others have reported de novo occurrence[910] and recurrence[8] of anti-GBM GN following SARS-CoV-2 infection, indicating a causative relation. Most patients with anti-GBM GN received plasma exchange, steroid, and either cyclophosphamide or rituximab or both. However, the outcome was poor, in that most patients either remained dialysis-dependent or died.[78910]
To date, six cases of AAV (anti-MPO: 3 and anti-PR3: 3) following SARS-CoV-2 infection have been reported.[3456] Five patients were treated with immunosuppression despite ongoing or recent infection with SARS-CoV-2 (rituximab: 2, cyclophosphamide: 3), and four patients showed improvement or stabilization of renal function. Rituximab was preferred when the risk of immunosuppression was considered high, whereas steroid and cytotoxic agents were used when the perceived risk of complications was low. Our patient tolerated immunosuppression and responded well, and showed partial renal recovery with the disappearance of both the autoantibodies following treatment.
Co-presentation of anti-GBM GN and AAV is rare, with anti-MPO antibody prevalence being more common than anti-PR3 antibody in them.[11] Double-positive patients predominantly shared characteristics of AAV such as old age, longer duration of symptoms before diagnosis, and features of anti-GBM GN such as lung hemorrhage. Renal survival in double-positive CrGN is better than anti-GBM GN, but inferior to AAV. Relapses are not uncommon in dual positive patients, whereas it is distinctly rare in anti-GBM GN.[11]
The pathogenesis of ANCA and anti-GBM antibody after SARS-CoV-2 infection is unclear. In addition to these autoantibodies, SARS-Cov-2 infection is reported to induce several others such as anti-nuclear antibodies and anti-phospholipid antibodies.[12] SARS-CoV-2 has a complex transcriptome and shares molecular similarities with human proteins and hence could generate various auto-antibodies.[12] The mechanism of association of concurrent occurrence of AAV and anti-GBM disease is unclear. There is some evidence that AAV may act as a trigger for anti-GBM disease[13] and vice versa.[14] The indolent course of CrGN, weak staining of linear IgG along the GBM on IF, a rapid disappearance of anti-GBM antibodies in serum after immunosuppression indicate that AAV is likely to be the dominant disease and anti-GBM disease is probably a secondary lesion in our patient. As SARS-CoV-2 infection is known to cause widespread vascular injury,[15] the appearance of these two autoantibodies independent of each other cannot be completely excluded. AVV and anti-GBM disease can appear during an active SARS-CoV-2 infection[3] or as sequelae of viral infection after several weeks[7] as seen in our case.
To our knowledge, this is the first reported case of “double antibody-positive” vasculitis as a post-SARS-CoV-2 sequela. An addition of autoimmune disease of the kidney to the ever-expanding plethora of clinical manifestations of SARS-CoV-2 infection is supported by our and recent few other case reports. We report this case to familiarize the clinicians regarding this rare phenomenon as these patients may have better renal survival if detected and treated early in the course of the disease.
Conclusions
To summarize, we present a case of severe renal failure and double-positive (ANCA and anti-GBM antibody) CrGN in a 59-year-old man as sequelae of SARS-CoV-2 infection. The patient responded well to the immunosuppression with preservation of renal function and disappearance of the autoantibodies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Incidence of acute kidney injury in COVID-19 infection: A systematic review and meta-analysis. Crit Care. 2020;24:346.
- [Google Scholar]
- Pathology of COVID-19-associated acute kidney injury. Clin Kidney J. 2021;14(Suppl 1):i30-9.
- [Google Scholar]
- De Novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5:2079-83.
- [Google Scholar]
- Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, A case report. Iran J Kidney Dis. 2020;14:239-42.
- [Google Scholar]
- Antineutrophil cytoplasmic antibody-associated glomerulonephritis in a case of scleroderma after recent diagnosis with COVID-19. Cureus. 2021;13:e12485.
- [Google Scholar]
- Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020;98:780-1.
- [Google Scholar]
- SARS-CoV-2 infection and recurrence of anti-glomerular basement disease: A case report. BMC Nephrol. 2021;22:75.
- [Google Scholar]
- Anti-glomerular basement membrane disease as a potential complication of COVID-19: A case report and review of literature. Cureus. 2020;12:e12089.
- [Google Scholar]
- Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959-68.
- [Google Scholar]
- Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92:693-702.
- [Google Scholar]
- SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci. 2021;14:898-907.
- [Google Scholar]
- Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol. 2011;22:1946-52.
- [Google Scholar]
- Autoantibodies against linear epitopes of myeloperoxidase in anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2016;11:568-75.
- [Google Scholar]
- COVID-19-associated vasculitis and vasculopathy. J Thromb Thrombolysis. 2020;50:499-511.
- [Google Scholar]







