Translate this page into:
Early Eosinophilic Antibody-mediated Rejection in a Renal Allograft Recipient
Address for correspondence: Dr. Sreejith Parameswaran, Department of Nephrology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. E-mail: sreejith.p@jipmer.edu.in
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Although the predominant component of acute allograft rejection is the T-cells, the milieu is not devoid of other inflammatory cells including plasma cells, eosinophils, and histiocytes. Apart from the CD8 T cell and CD4 T cell-FasL cytotoxicity, experimental models had proven a pivotal role of Th-2 cells in acute rejection, and these have been associated with marked tissue eosinophilia. Herein, we present a unique case of severe eosinophilic acute antibody-mediated rejection in a 22 years old deceased donor renal allograft recipient, within 4 days of transplantation without peripheral eosinophilia. The pathology was successfully dealt with the prevailing modalities of therapy, including steroids, plasmapheresis, intravenous immunoglobulin, and bortezomib. Concurrently, we have briefly reviewed the literature about the role of eosinophils in graft rejection and its prognostication.
Keywords
Antibody-mediated rejection
cluster designation
intravenous immunoglobulin
peritubular capillaritis
Introduction
Eosinophils traditionally considered weakly phagocytic has potential immunoregulatory attributes. There has been innumerable literature on eosinophils in inflammatory exudates of rejected allografts. After induction of production and differentiation by Th-2 cytokines, and later augmented by other interleukins (IL-5), eosinophils release an array of cationic proteins and growth factors, which trigger a cascade of vascular and parenchymal injury.[1] Herein, we present an unusual case of acute antibody mediated rejection with significant graft eosinophilia.
Case Report
A 22-year-old man underwent deceased donor kidney transplantation at our center in December 2015 after remaining on dialysis for 9 months. He had not received any blood transfusions or transplant in the past, and no panel reactive antibody was assessed before transplant. Donor was a 34-year-old female with no comorbidities and a frozen section of donor kidney before harvesting showed normal histology. The cold ischemia time was 12 h. He received induction with rabbit anti-thymocyte globulin (2 mg/kg) before engraftment. Post procedure, urine output was 100–150 mL/h, and he was hemodynamically stable with a pulse rate of 92 per min, blood pressure of 150/90 mmHg, and temperature of 37.8°. Twelve hours later, he had a serum creatinine of 5.8 mg/dL, hemoglobin 7.5 g/dL, drain output of 600 mL and had passed more than 1500 mL of urine. Over the next 4 days, serum creatinine gradually reduced to 3.0 mg/dL. He was on standard triple drug immunosuppression of tacrolimus, mycophenolate mofetil, and prednisolone.
Fifth-day posttransplant there was fall in urine output to 50 mL/h, and serum creatinine rose to 4.1 mg/dL. Concurrently, hemoglobin was 12 g/dl, total count 10500 per cu.mm with a differential count of 86% neutrophils, 8% lymphocytes, and 6% monocytes with no aberrant findings in peripheral smear. He was given 500 mg of intravenous methylprednisolone for 3 days, a graft biopsy performed with a high suspicion of rejection, and thereafter initiated on plasma exchange and intravenous immunoglobulin (IVIG) therapy.
The histopathology of the graft showed [Figure 1] 15 glomeruli, and significant interstitial inflammation with lymphocytes and numerous eosinophils. There was evidence of glomerulitis (Banff g1) and 20% acute tubular necrosis (ATN). Peritubular capillaries (PTC) were infiltrated with lymphocytes, eosinophils, and few plasma cells amounting to Banff peritubular capillaritis score 3 (ptc3). Immunofluorescent staining showed strongly positive C4d in PTC. The tissue was abound with eosinophils effectuating eosinophilic glomerulitis, interstitial nephritis, and peritubular capillaritis. Graft eosinophilia accounted for more than 15% of the interstitial infiltrates. Eosinophils were farther from the site of ATN, and there was no evidence of tubulitis. These findings were consistent with an eosinophil-rich acute antibody-mediated rejection.

- Histopathology of renal allograft renal biopsy on 7th day posttransplant. (a) Interstitial infiltration of lymphocytes admixed with numerous eosinophils (H and E, ×200); (b) eosinophil infiltration within capillary loops causing glomerulitis (H and E, ×200); (c) peritubular capillary infiltration by eosinophils (PAS, ×200); (d) Immunohistochemistry with C4d shows diffuse strong positivity along peritubular capillaries and basement membrane (immunohistochemistry C4d, ×200)
He received a total of 7 sessions of plasma exchange and 75 g of IVIG followed by bortezomib 1.3 mg/m2/dose for 4 doses (day 1, 4, 7, 11). The graft function improved with urine output of 4 l/day and serum creatinine reaching 1.9 mg/dl at 17 days posttransplant. Thereupon, a repeat graft biopsy was performed [Figure 2a and b]. The specimen was inadequate with 2 glomeruli but had a long segment of medulla showing resolution of the inflammation and Banff ptc1. Circumferential positivity of C4d in PTC persisted (Banff score – g0, t0, i1, v0, cg0, ct0, ci0, cv0, aah0, ptc1, C4d3).
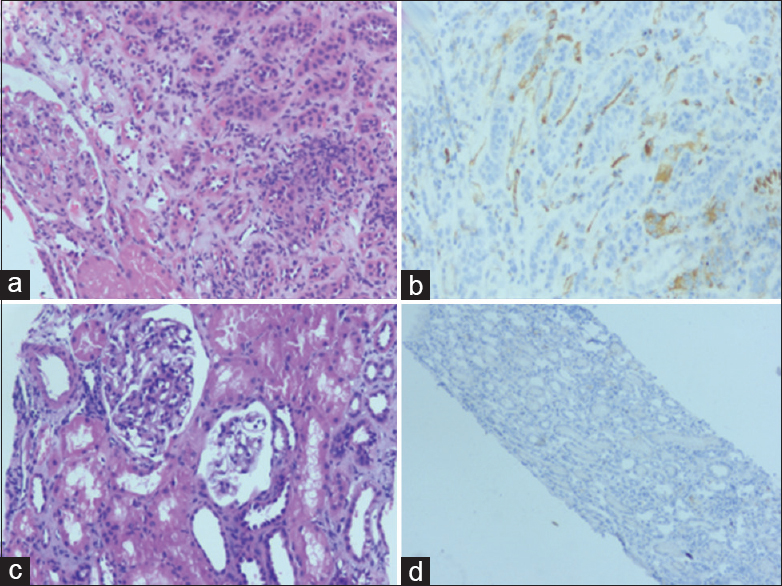
- Day 20 posttransplant. (a) Shows peritubular capillary infiltration by lymphocytes (H and E, ×100). (b) Immunohistochemistry with C4d shows strong positivity along many peritubular capillaries (immunohistochemistry C4d, ×200); and day 90 posttransplant. (c) Normal glomeruli with no evidence of glomerulitis (H and E, ×100). (d) Immunohistochemistry with C4d reveals no staining along peritubular capillaries (immunohistochemistry C4d, ×100)
Ninety days after transplant, a third biopsy was carried out which revealed 8 glomeruli of which 3 were sclerosed [Figure 2c and d]. Nevertheless, there were no tubulitis, interstitial inflammation, peritubular capillaritis, glomerulitis, or C4d positivity. Four months posttransplant, his serum creatinine is 1.6 mg/dL.
Discussion
We report a case of acute antibody mediated graft rejection following a deceased donor kidney transplantation, which had eosinophil-rich interstitial infiltration (eosinophilic Antibody-mediated rejection [AMR]). Acute graft rejection with eosinophils rich inflammation is rare and is usually associated with poor graft outcomes. However, our patient responded well with standard treatment for AMR.
Distinct from previous postulates, eosinophils is now considered a true immunoregulatory cell with roles in antigen presentation, B-cell priming, and regulation of T cells, dendritic cells, mast cells, basophils, and neutrophils.[2] The presence of eosinophils in acute rejection had been underestimated due to the conventional staining techniques. Immunohistochemical localization of major basic protein and eosinophilic cationic protein and epifluorescence techniques have attested the eosinophilic infiltration in renal interstitium and arteries amid acute rejection.[3]
Apart from the direct cytotoxicity which mediate graft rejection, CD8 T cells can prevent eosinophilic graft rejection. In the absence of CD8 T cells, there is sustained production of TH2 cytokines by CD4 T cells, IL-4 and IL-13, which upregulate VCAMs (vascular cell adhesion molecule) and eotaxin and finally recruit the eosinophils, boosted by IL-5 and IL-9. Resultantly, when the CD8 T cell pathway and CD95L–CD95 pathway of CD4+T-cell-mediated rejection are impeded, the IL-5 eosinophil pathway of rejection becomes pivotal.[1] In allograft rejection, the antigens that are large and unable to pass through the capillaries, draw the inflammatory cells to the site, and such antigens are likely to evoke an eosinophilic response from the chemotactic factors released by T cells.[45] The experimental model of mouse cardiac allograft had proven the role of TH-2 cells in acute allograft rejection, which in turn exhibits marked eosinophil infiltration.[6] Vascular injury and inflammation express abundant eotaxin, leukotriene-B4 and VCAMs attracting the eosinophils in these sites.[13]
Eosinophils contain three types of granules (primary granules, specific or secondary granules, and small granules) and possess receptors for several cytokines, including interleukin lL-3, IL-5, and granulocyte-macrophage-colony-stimulating factor, which mediate colony formation. The unique role of IL-5 in the production, activation, and localization of eosinophils makes it a prime target for therapeutic intervention.[7] In other scenarios associated with eosinophilia, there is striking increase in light density eosinophils (density <1.082), which are metabolically more active, generate abundant superoxide ions and has potent cytotoxicity for antibody-coated targets.[8]
Eosinophils once activated, release a variety of cytotoxic products, including major basic protein, which is correlated with increased IL-2 and IL-2R production, eosinophilic cationic protein which can modulate lymphocyte responses and cause cell membrane damage, and other chemical mediators (such as peroxidase, phospholipase, arylsulphatase, LTC-4, and 15-HETE) which modify the inflammatory responses and promote fibrogenesis (implied in chronic allograft injury).[57]
Although eosinophilic infiltration represents a remarkable positive prognostic indicator in tumors (lung, gastric, Hodgkin's disease), it has not been so with renal allograft.[910] The role and prognostic significance of eosinophils in graft have not been fully elucidated. Few studies have even implied a poor graft outcome or corticosteroid resistant rejection. Interstitial infiltrates of eosinophils can be seen in 30% of biopsies with acute rejection and is usually <2%–3% of the infiltrates. The 4% cutoff value for tissue eosinophilia, as an insignia of poor outcome in graft rejection, put forth by Weir et al., was further endorsed by Kormendi et al. Such a magnitude of tissue eosinophilia had 78% sensitivity and 91% specificity for serious and irreversible rejection. As the rejection pathology tends to be focal, the tissue eosinophil density may be a more reliable parameter to assess than the percentage of eosinophils. Median tissue eosinophil density was found to be higher in grafts with acute rejection or graft loss compared to nonrejected grafts.[41112]
There is also a discrepancy with regard to the degree of eosinophilia and the intensity of rejection. Nevertheless, unsampled acute vascular rejection can be contemplated in any unexplained graft eosinophilia. Prominent eosinophils may indicate the presence of transplant endarteritis which implies Banff category 4 Type 2 rejection.[1314] In the Meleg-Smith and Gauthier study, 13 allografts with vascular rejection had an average of 20% graft interstitial eosinophil infiltrates compared to <1% in those without vascular rejection.[13]
Pertinent to this entity is the peripheral blood eosinophilia. Both graft eosinophilia and peripheral eosinophilia have low sensitivity but high specificity (>90%) in predicting acute rejection. Furthermore, peripheral blood eosinophilia was found to be higher (1.5%–3%) in different types of acute cellular rejection compared to the controls. Rise of blood eosinophils in early months of transplantation can be a harbinger of graft rejection.[12] However, in the index case, peripheral eosinophilia was conspicuously absent, for which the author could not find any supporting literature.
In an eosinophil-rich interstitial inflammation, drug-induced allergic interstitial nephritis is considered if eosinophils invade the tubules, has eosinophilic casts, is predominantly centered on the corticomedullary junction (not confined to the cortex) and PTC C4d is negative.[15] In this case, there was interstitial inflammation, glomerulitis, peritubular capillaritis with no evidence of tubulitis. The presence of microcirculatory inflammation and PTC C4d positivity favor a diagnosis of acute AMR with eosinophilic infiltrate. In spite of marked graft eosinophilia and severe AMR, the pathology had responded to timely steroid pulse, plasmapheresis, IVIG followed by bortezomib.
Conclusion
In the realm of graft rejection, the relevance of eosinophil and its prognostication has been elucidated in several case studies. Significant graft tissue eosinophilia has been strongly associated with vascular rejection and poor graft outcome, while blood eosinophilia also may portend graft rejection. Notwithstanding, the index case, first reported case of eosinophilic AMR, responded promptly to conventional therapies of acute AMR. Once sidelined, the role of TH2 cells and eosinophils in allograft pathology need to be henceforth reconsidered.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Immunoregulatory roles of eosinophils: A new look at a familiar cell. Clin Exp Allergy. 2008;38:1254-63.
- [Google Scholar]
- Role of the eosinophil in chronic vascular rejection of renal allografts. Am J Kidney Dis. 1995;26:634-42.
- [Google Scholar]
- The prognostic value of the eosinophil in acute renal allograft rejection. Transplantation. 1986;41:709-12.
- [Google Scholar]
- Lymphocyte products. In: Mahmoud AA, Austen KF, Simon AS, eds. The Eosinophil in Health and Disease. New York: Grune and Stratton; 1980. p. :293.
- [Google Scholar]
- Heterogeneity of T cell clones specific for a single indirect alloantigenic epitope (I-Ab/H-2Kd54-68) that mediate transplant rejection. Transplantation. 2000;70:1516-24.
- [Google Scholar]
- Ultrastructural study of eosinophils from patients with the hypereosinophilic syndrome: A morphological basis of hypodense eosinophils. Blood. 1988;71:780-5.
- [Google Scholar]
- Renal allograft eosinophilia: An unusual presentation of sudden graft dysfunction. Indian J Nephrol. 2017;27:129-30.
- [Google Scholar]
- The importance of eosinophil cells in kidney allograft rejection. Transplantation. 1988;45:537-9.
- [Google Scholar]
- Blood and graft eosinophilia as a rejection index in kidney transplant. Nephron. 1993;65:304-9.
- [Google Scholar]
- Abundance of interstitial eosinophils in renal allografts is associated with vascular rejection. Transplantation. 2005;79:444-50.
- [Google Scholar]
- Significance of interstitial lesions as the early indicator for acute vascular rejection in human renal allografts. Clin Transplant. 1999;13(Suppl 1):17-23.
- [Google Scholar]
- Renal transplant pathology. In: Hepinstall Pathology of Kidney (7th ed). Philadelphia: Lippincott Williams & Wilkins; 2014. p. :1333.:1420. Ch. 29
- [Google Scholar]







