Translate this page into:
Effect of Chronotherapy of Antihypertensives in Chronic Kidney Disease: A Randomized Control Trial
Address for correspondence: Dr. Vaibhav Tiwari, Department of Nephrology, Sir Ganga Ram Hospital, Old Rajinder Nagar, New Delhi - 110 060, India. E-mail: drvt87@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There is a higher prevalence of non-dipping pattern in hypertensive chronic kidney disease (CKD) patients. Nocturnal hypertension has been shown to predict cardiovascular mortality and morbidity and is often superior to daytime blood pressure. We studied the effect of shifting or adding antihypertensive to night time on blood pressure profile of CKD III-IV patients.
Methods:
In this single-center, prospective, randomized controlled trial, eligible participants were adults from eastern India aged 18–65 years with CKD stages 3 and 4, with a non-dipping pattern on ambulatory blood pressure monitor (ABPM). The intervention group received all the antihypertensives in the night time whereas the standard care group continued to take the medication in the morning. Both groups were followed up for 1 year. The primary outcome was the number of patients changed from non-dippers to dippers in the standard care group and intervention group. Secondary outcomes included a change in estimated glomerular filtration rate (eGFR) and change in the cardiac structure.
Results:
39 patients in the intervention group and 36 patients in the standard care group were analyzed. 10 patients (26%) reverted to dipping pattern in the intervention group as compared to none in the standard care group. Mean changes in eGFR were −2.55 and −0.18 mL/min/1.73 m2 in the standard care and intervention group at the end of the study, respectively. Between-group difference in eGFR was significant at 1 year (5.22 [95% CI, 4.3–6.1] ml/min/1.73 m2); (P = 0.03). The cardiac structure showed no significant changes in either group.
Conclusions:
Bedtime administration of antihypertensives reverted non-dippers to dippers and slowed the decline in eGFR in CKD stages 3 and 4 compared to morning administration of antihypertensives.
Keywords
ABPM
antihypertensives
chronic kidney disease
dippers
hypertension
nocturnal hypertension
non-dippers
Introduction
Hypertension is both a cause and an effect of chronic kidney disease (CKD). Hypertension is seen in at least 70% of people with CKD when measured using standard methods.[1].
Approximately one in three adults in India has hypertension.[2] The prevalence of hypertension is higher among patients with CKD, progressively increasing with the severity of CKD. Data from Indian screening and early evaluation of kidney disease show that the prevalence of hypertension and CKD in the SEEK cohort are 43.5 and 17.2%, respectively. The prevalence of hypertension in CKD exceeds that of the general population (64.5% in CKD population vs 41.9% in the non-CKD population).[3] Data from MDRD study, for example, showed that the prevalence of hypertension rose progressively from 65 to 95%, as the GFR fell from 85 to 15 mL/min per 1.73 m2.[4].
In healthy individuals, there is a diurnal variation in blood pressure (BP) such that BP decreases by 10–20% during the night time compared with the daytime, which is known as the dipping pattern. Failure of the blood pressure to fall by at least 10% during sleep is called a non-dipping pattern. CKD has been associated with a higher prevalence of the non-dipping pattern. Many studies have indicated that the non-dipping BP pattern in CKD patients results in significant target organ damage such as subsequent faster deterioration in renal function with more renal morphological changes, high incidence of cardiovascular diseases (CVD) as well as it is an early predictor of nephropathy in type 1 diabetes, and poor long-term survival in hemodialysis patients.
The time of ingestion of antihypertensive medications can affect the circadian pattern of BP, independent of the terminal half-life of each individual medication. Treatment at bedtime as shown in studies on patients with essential hypertension[5678910111213] can be a cost-effective and simple strategy for successfully achieving therapeutic goals of adequate BP reduction during sleep and preserving or re-establishing normal 24-h BP dipping pattern.
Whether this is true for patients with hypertension and CKD and if it impacts end-organ damage is not well studied. This study was conducted to study the effect of nocturnal dosing of antihypertensives on blood pressure variation especially during sleep and to study its clinical impact on renal and cardiovascular outcomes in patients with CKD.
Methods
This was a single-center, prospective, randomized controlled study conducted in eastern India. The study was approved by the IPGMER Research Oversight Committee (approval number- Inst/IEC/2016/432). The participants gave written informed consent in accordance with the principles of the Declaration of Helsinki. (CTRI/2018/01/011581).
Eligible participants were patients of both sexes aged 18 to 65 years with an estimated glomerular filtration rate (eGFR) of 15 to 60 mL/min/1.73 m2 (as calculated with the 4-variable MDRD [modification of diet in renal disease] study equation). Patients on antihypertensives taking ≥ one drug(s) of which at least one dose is being taken in the morning or afternoon were included. Exclusion criteria were patients with secondary hypertension other than that due to kidney disease, uncontrolled hypertension (>150/90), pregnancy, recent myocardial infarction, stroke, and pregnancy. The study was carried out at IPGMER, a government-run tertiary-care center in Kolkata, India. The total study duration was 21 months from February 2016 through December 2017.
Ambulatory blood pressure monitor (ABPM) was carried out with an appropriately calibrated NISSEI model: DS-250 (NIHON SEIMITSU SOKKI CO., LTD.). Systolic BP (SBP) and diastolic BP (DBP) were automatically measured and recorded every 20 min during the day and every 30 min at night. Patients were instructed to fill in a diary of activities indicating the times of getting up and going to bed, in order to determine later the activity and rest periods, as well as any other incidences which could affect their BP. Patients in whom ambulatory BP monitoring showed <70% of valid BP readings or more than three consecutive hours without any BP measurement were excluded from the study. Patients who developed any complications requiring stopping of antihypertensives were excluded from the analysis.
Eligible patients underwent ABPM at the initiation. Dippers were excluded from the study. Patients were randomly assigned to either the standard or intervention group. The standard care group continued taking medication as previously. In the intervention group, all antihypertensives except diuretics were shifted to night time. Additional antihypertensives were added if required as per treating physician's discretion in the morning and at night time in the standard care group and intervention group, respectively. Patients underwent repeat ABPM study at the end of 12 weeks. Dipping was defined by sleep time relative systolic BP declined ≥ 10%. It was calculated as ([awake BP mean − asleep BP mean)/awake BP mean) × 100. Antihypertensives were continued throughout the study period. Patients were evaluated at baseline and every 3 months for history, physical examination, assessment of any adverse events or endpoints, serum creatinine levels, urine examination, and any other tests as needed by the treating clinician.
The primary outcome was the proportion of patients reverted back to dipping pattern in both the groups. Secondary outcome measures were changes in eGFR in the two groups from baseline and at the end of 1-year and cardiac structure changes as assessed by 2D ECHO. A computer-generated random-number table was used for the allocation of individuals in the intervention group and the control group in a 1:1 ratio.
Summary statistics of numeric variables were expressed as mean ± standard deviation, and categorical data, as proportion. Baseline variables were compared between groups to detect any variability at baseline. To determine differences between groups of categorical data Fisher exact test or Pearson x2 test were used as applicable. Between-group comparison of numeric parametric data was done by unpaired t-test. Within-group repeated-measure parametric data were compared for statistical significance using repeated-measure analysis of variance. The 95% confidence interval (CI) of the difference between mean values was determined and P ≤ 0.05 was considered statistically significant.
Sample size power calculation
We estimated that a minimum of 23 patients was necessary to detect a decrease in the SBP after chronotherapy of at least 6 mmHg using an unpaired t-test with one-tailed α = 0.05 and a power of 0.80 assuming a standard deviation of the differences of 11 mmHg based on previous observations by other groups.[14].
Results
A total of 96 patients were screened for initial enrolment based on clinically controlled BP (<140/90) on an outpatient basis. 39 patients were assigned in the intervention group and 36 remained in the standard care group. All patients completed the study and were available for final analysis [Figure 1].

- Flowchart shows the number of subjects entering the study from enrolment, allocation, and follow-up
Patients were recruited from February 2016 to December 2016 and follow up of the last patient ended in December 2017. The baseline characteristics of patients are presented in Table 1. There were no significant differences in intervention and standard care groups with respect to baseline variables. The average number of antihypertensives was 2.58 in the intervention group and 2.67 in standard care group with amlodipine used most commonly in both the groups. Torsemide was used as the most common diuretic and Telmisartan as the most common ARB in both groups.
| Variables (Mean) | Standard care group | Intervention group | P |
|---|---|---|---|
| Patients, n | 36 | 39 | |
| Sex- Male (%) | 11 (30.56%) | 12 (30.77%) | 0.98 |
| Diabetes (%) | 15 (41.67%) | 17 (43.59%) | 0.87 |
| Age (years) | 52.86±13.01 | 51.15±12.06 | 0.51 |
| BMI, kg/m2 | 22.79±1.8 | 22.54±1.51 | 0.35 |
| Office systolic BP, mmHg | 130.32±11.23 | 132.14±10.51 | 0.77 |
| Office diastolic BP, mmHg | 74.67±8.36 | 76.78±9.82 | 0.38 |
| Office pulse rate, | 77.54±10.24 | 74.64±10.84 | 0.88 |
| Creatinine, mg/dL | 2.32±0.68 | 2.18±0.58 | 0.35 |
| eGFR, mL/min/1.732 m2 | 32.46±13.42 | 34.59±12.43 | 0.32 |
| No. of hypertension medications | 2.58±1.1 | 2.67±0.96 | 0.81 |
| Systolic BP, mmHg | |||
| Awake | 131.64±13.13 | 133.31±13.47 | 0.53 |
| Asleep | 124.94±13.14 | 126.49±13.83 | 0.48 |
| 24 h | 128.72±13.29 | 130.92±13.21 | 0.39 |
| Diastolic BP, mmHg | |||
| Awake | 84.36±11.67 | 83.21±11.67 | 0.64 |
| Asleep | 76.31±12.55 | 78.36±13.67 | 0.70 |
| 24 h | 79.28±11.43 | 80.59±11.24 | 0.60 |
| Sleep time relative BP decline, % | 5.10±2.61 | 5.08±4.52 | 0.27 |
| Left ventricular index, gm/m2 | 124.19±13.91 | 127.15±13.91 | 0.47 |
| Ejection fraction, % | 60.64±5.87 | 60.21±8.02 | 0.96 |
On repeat ABPM at 12 weeks as shown in Table 2, Asleep systolic BP showed a reduction of −6.44 mmHg (P = 0.02) and asleep DBP showed −3.57 mmHg reduction (P = 0.03) in Intervention group compared to the standard care group. 24 h mean SBP variations were not significant between the two groups (P = 0.43). BP variations over 24 h of both groups have been shown in [Figure 2a and b. Daytime SBP showed a non-significant reduction of −2.46 mmHg and −2.78 mmHg in intervention and standard care group, respectively (P = 0.19). Daytime DBP also showed non-significant reduction −0.77 and −1.86 mmHg in the intervention and standard care group, respectively. Reversion to dipping from non-dipping patterns occurred in 10 (25.6%) patients in the Intervention group whereas none of the patients in the Standard care group reverted to dipping pattern (P < 0.01). The mean sleep time relative systolic BP decline increased from 5.08 ± 4.52% to 8.30 ± 4.34% (P = 0.009) in the intervention group whereas there was a non-significant increase from 5.10 ± 2.61% to 5.93 ± 2.36% (P = 0.79) in the standard care group.
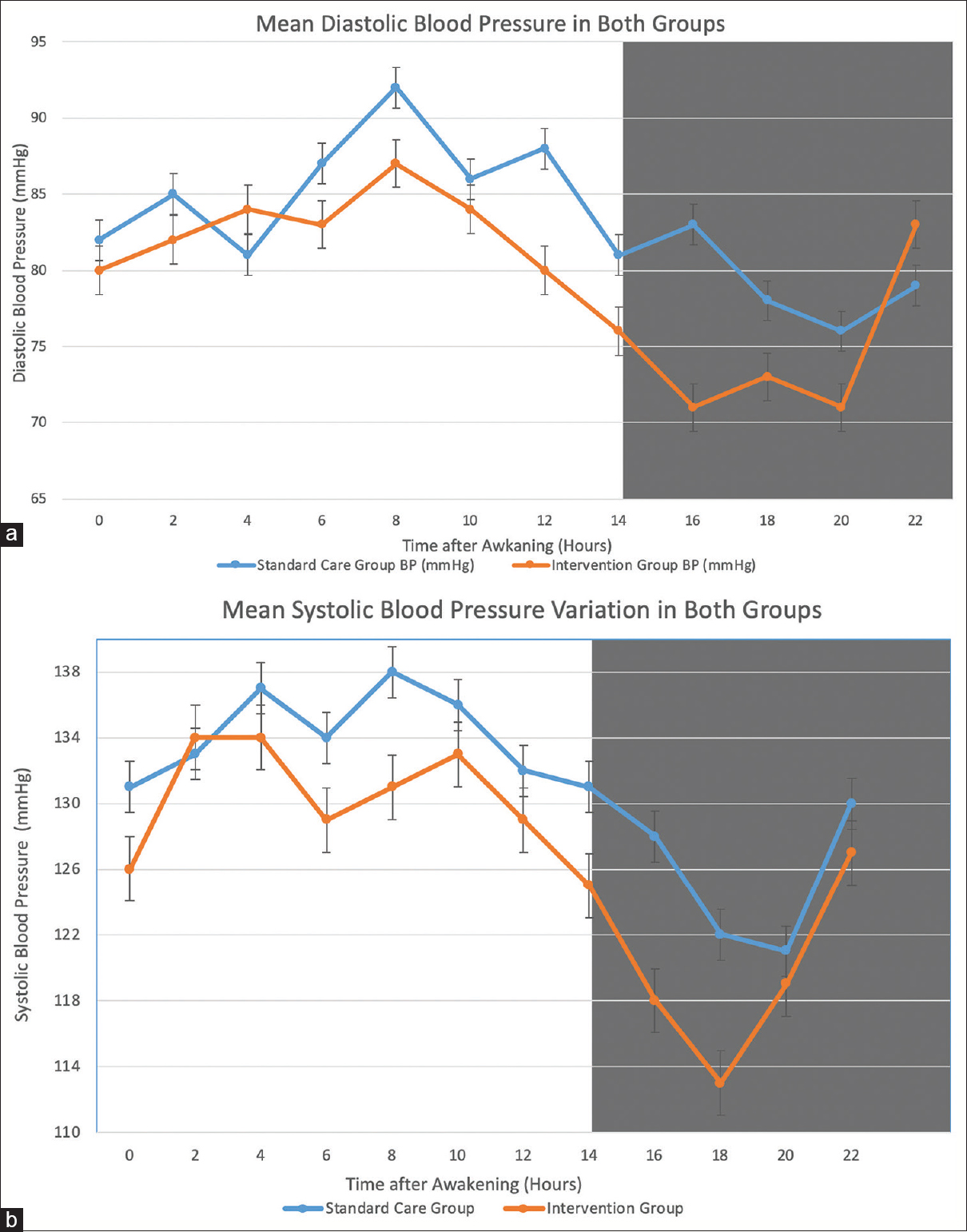
- (a and b) Changes in the circadian pattern of SBP and DBP between Intervention and Standard care group in CKD patients sampled 24-h ABPM. Each graph shows the 2 hourly means and SEs of data collected from the standard care group (Blue line) and intervention group (Orange line) after 12 weeks of treatment. Dark shading along the graphs represents the average hours of nocturnal sleep across the patient sample
Changes in eGFR in the standard care and intervention groups over 12 months are presented in Table 3. Mean eGFR in intervention group showed a non-significant decrease from 34.59 ± 12.43 mL/min/1.73 m2 to 34.41 ± 11.82 mL/min/1.73 m2. In the standard care group, eGFR decreased significantly from 32.46 ± 13.42 mL/min/1.73 m2 to 29.91 ± 9.97 mL/min/1.73 m2 over the same period (P = 0.03). Mean changes in eGFR were −2.55 and −0.18 mL/min/1.73 m2 in the standard care and intervention group, respectively.
| Variables (Mean) | Standard care group | Intervention group | P |
|---|---|---|---|
| Systolic BP, mmHg | |||
| Awake | 134.42 ± 5.98 | 130.85 ± 11.9 | 0.25 |
| Asleep | 126.47 ± 7.14 | 120.03 ± 11.85 | 0.02 |
| 24 hours | 129.83 ± 8.02 | 126.92 ± 12.23 | 0.43 |
| Diastolic BP, mmHg | |||
| Awake | 86.22 ± 6.81 | 82.44 ± 8.88 | 0.02 |
| Asleep | 79.44 ± 6.8 | 75.87 ± 9.13 | 0.03 |
| 24 hours | 82.25 ± 6.81 | 79.10 ± 8.81 | 0.07 |
| Sleep time relative BP decline, % | 5.93 ± 2.36 | 8.30 ± 4.34 | 0.01 |
| Dipper, number of patient | 0 | 10 | <0.001 |
| Variables (Mean) | Standard care group | Intervention group | P |
|---|---|---|---|
| Patients, n | 36 | 39 | |
| Office systolic BP, mmHg | 132.32 ± 15.64 | 129.84 ± 15.58 | 0.69 |
| Office diastolic BP, mmHg | 76.97 ± 6.39 | 74.77 ± 4.72 | 0.53 |
| Office pulse rate | 75.84 ± 8.29 | 72.24 ± 12.34 | 0.77 |
| Creatinine, mg/dL | 2.44 ± 0.64 | 2.2 ± 0.57 | 0.09 |
| eGFR, mL/min/1.732 m2 | 29.91 ± 9.97 | 34.41 ± 11.82 | 0.03 |
| No. of hypertension medications | 2.8 ± 0.74 | 2.72 ± 0.85 | 0.70 |
| Left ventricular index, gm/m2 | 125.52 ± 13.49 | 125.54 ± 13.9 | 0.39 |
| Ejection fraction % | 60.83 ± 5.86 | 60.46 ± 6.36 | 0.43 |
Cardiac structural changes did not show any significant changes in either group. Left ventricular (LV) mass index in intervention group decreased by mean 1.61 gm/m2 (P = 0.17), it increased by 1.33 gm/m2 in standard care group (P = 0.385). The between-group difference in LV mass index was non-significant at 1 year (0.02, 95% CI − 1.02–1.06] gm/m2). None of the patients had any major cardiovascular events, death due to any cause or development of CKD stage V.
Discussion
This study provides evidence that the non-dipping pattern in CKD patients is reversible and can be achieved by shifting the antihypertensives to night time. Many studies have shown previously the benefits of night-time antihypertensives on nocturnal BP and dipping pattern but most studies were done in normal subjects with essential hypertension and the few studies done on CKD patients did not have a significant number of those with severe renal dysfunction. This trial has included advanced CKD and was expecting a higher event rate. CKD patients differ from normal population in hypertension in regards to abnormal salt water retention, overactive sympathetic drive and over activation of the renin-angiotensin-aldosterone system. The first study to demonstrate the alteration BP profile by the administration of night-time antihypertensives in CKD was done by Portaluppi et al. which showed a significant fall of nocturnal BP.[15] It was an uncontrolled study with 16 patients and did not report the number of dippers reverted from a non-dipping pattern. In an uncontrolled study by Minutolo et al. 32 patients with CKD were subjected to night-time antihypertensives.[16] They reported a reversion of 88% patients to dipping pattern as compared to our study which has shown a 26% reversion. This may be due to well-controlled BP at baseline as per ABPM criteria in that study with nocturnal systolic BP 114 ± 11 mmHg and diastolic BP 62 ± 7 mmHg and 90% of patients were in CKD stage II and III. In a large randomized study in Spain by Hermida et al. it was seen that night-time antihypertensives provided better eGFR preservation, proteinuria reduction, CVD outcomes and reversion of 24% of patients to dipping pattern.[17] Our findings closely match with this large trial but in the present trial neither there were CVD events in either group nor significant changes in cardiac structure in both the group. This may be due to the early stage of CKD patients in Hermida et al. study that permitted higher use of ACEi/ARBs which we could not use as significant hyperkalemia in our patients precluded the usage. Another reason may be the long follow up of 5.7 years in Hermida et al. as compared to 1 year in this study. In another randomized controlled trial by Wang et al., a positive correlation was seen with nocturnal antihypertensives in proteinuria reduction, prevention of deterioration of Left ventricle mass Index and reversion of 80% patient to dipping pattern.[18] Again, this study had a mean eGFR of 70 ± 26 mL/min/1.73 m2 and well-controlled BP at baseline with nocturnal SBP 124 ± 12 mmHg and diastolic BP 75 ± 13 mmHg.
Different studies have used different classes of antihypertensives for nocturnal administration, maintaining the daytime efficacy as well with the night dose, particularly reducing nocturnal BP and restoring dipping pattern. In a study by Hermida et al., different combinations of valsartan and amlodipine were studied in relation to the timing of administration.[19] The maximum benefit was found when both the drugs were given during the night time. In the present study, we aimed to see a reversal to dipping pattern and its impact on outcomes and since it is difficult to predict the number of drugs needed to restore the dipping pattern, all the antihypertensives were shifted to night dose other than diuretics for maximal effect.
On the basis of the findings of the present study, we recommend the shifting of all medication except the diuretics in the night time. This can be followed up by an ABPM to assess the BP profile and adjusting the medication accordingly or daytime BP monitoring and adding another antihypertensive during the day.
In our study 80% of patients had eGFR <45 mL/min/1.73 m2 with 50% having eGFR 15–30 mL/min/1.732 m2. As was shown in previous studies there is a proportional rise in the prevalence of non-dippers with increasing severity of CKD. All previous studies had patients in the early stages of CKD with few in stage IV CKD. Thus, it was uncertain that if reversal to physiological Blood pressure was achievable with more severe disease. In the present trial, 40% of patients belong to stage IV CKD with a mean eGFR 23.01 ± 2.71 mL/min/1.73 m2. Since the eGFR was low, ACEi/ARBs could not be used liberally and more than 60% of patients in each group were not taking ACEi/ARBs at the end of the study. Despite that, the intervention group had shown slower eGFR decline than the standard care group. Since most of the characteristics were similar in both the groups, better night-time BP control may have a role to play in the effective preservation of renal function. The decline in GFR heralds the loss of diurnal variation in BP. However, vice-versa although seems plausible, has not been elucidated fully. The loss of diurnal variation is associated with hypertension, more cumulative end-organ damage, sympathetic over-activity, insulin resistance, salt-sensitivity, sleep apnea, and endothelial dysfunction can themselves cause worsening of renal function.[202122232425] It has been suggested that the processes that cause non-dipping also cause CKD progression. And these may be a concurrently occurring phenomenon. However, the preservation of GFR by changing dipping status is a pointer toward its causal association. The exact pathophysiological mechanisms to confirm this renal preservation effect of reversal to dipping pattern in CKD needs further studies.
When compared to the day time BP in both the groups, the intervention group had better although not significant SBP reduction (131 mmHg in the intervention vs 134 mmHg in standard care group) whereas there was a significant reduction in DBP (82 mmHg in the intervention group vs 86 mmHg in standard care group), it shows that the efficacy of BP-lowering medication did not get dissipated to control the BP over extended periods and had even significantly better control of DBP. This effect was probably independent of the class of drugs since most patients were on different antihypertensives. Cardiac structure changes showed a 1.26% decrease whereas a 1.1% increase from baseline in intervention and standard care group, respectively. Most of the patients have had higher mean LV mass index values than the general population and patients when compared to a similar duration of hypertension without CKD. This may have translated into a severe degree of non-reversible changes resistant to the effect of controlled BP or nocturnal dipping. Another possibility is the short follow up period of 52 weeks which may be insufficient for showing the effect.
In the present study, ABPM was repeated at 3 months after the introduction of the night time dose of antihypertensives. Although there was a reversion to dippers in 26% of patients, the benefit was seen in the whole group at the end of 1 year. Possibility of picking up more dippers if the ABPM was repeated at 6 or 9 months, is high. Therefore, further studies with more frequent monitoring are required to get an additional number of patients who would have become dippers in the due course.
Strengths of the study: It was adequately powered to detect reversion to dipping pattern, the inclusion of patients with severe renal dysfunction and no dropouts for the reason of inadequate ABPM values over a 24-h period. Our study has some potential limitations. Short follow up period and limited sample size have restricted our results for generalizability as we have not documented any cardiac events which are the most common cause of morbidity and mortality in the CKD population. A more frequent ABPM would have provided a better measure of the control of nocturnal hypertension as well as helped us to pick up additional dippers.
Conclusions
Our results indicate that in patients with severe renal dysfunction, nocturnal antihypertensive dosing can achieve a reversal to dipping pattern. A simple shift of bedtime administration of antihypertensives in the CKD population, even with severe renal dysfunction (CKD 3 or 4) results in restoration of the circadian blood pressure variability and provides renal protection by slowing the deterioration of eGFR. Though we could not demonstrate a further cardiovascular benefit, trials with longer follow up are needed to ascertain it.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Prevalence of high blood pressure and elevated serum creatinine level in the United States: Findings from the third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161:1207-16.
- [Google Scholar]
- Hypertension in India: A systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170-7.
- [Google Scholar]
- Epidemiology and risk factors of chronic kidney disease in India – results from the SEEK (Screening and early evaluation of kidney disease) study. BMC Nephrol. 2013;14:114.
- [Google Scholar]
- Prevalence of hypertension in 1,795 subjects with chronic renal disease: The modification of diet in renal disease study baseline cohort. Am J Kidney Dis. 1996;28(6):811-21.
- [Google Scholar]
- Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens. 2011;24:383-91.
- [Google Scholar]
- Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: Improved blood pressure control with bedtime dosing. Hypertension. 2009;54:40-6.
- [Google Scholar]
- Chronotherapy of hypertension: Administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59:923-39.
- [Google Scholar]
- Administration-time differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int. 2013;30:280-314.
- [Google Scholar]
- Administration-time-dependent effects of spirapril on ambulatory blood pressure in uncomplicated essential hypertension. Chronobiol Int. 2010;27:560-74.
- [Google Scholar]
- Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int. 2009;26:61-79.
- [Google Scholar]
- Effect of dosing time of angiotensin II receptor blockade titrated by self-measured blood pressure recordings on cardiorenal protection in hypertensives: The Japan morning surge-target organ protection (J-TOP) study. J Hypertens. 2010;28:1574-83.
- [Google Scholar]
- Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50:715-22.
- [Google Scholar]
- Chronotherapy with nifedipine GITS in hypertensive patients: Improved efficacy and safety with bedtime dosing. Am J Hypertens. 2008;21:948-54.
- [Google Scholar]
- Reproducibility of ambulatory blood pressure in treated and untreated hypertensive patients. J Hypertens. 2010;28:918-24.
- [Google Scholar]
- Altered circadian rhythms of blood pressure and heart rate in non-hemodialysis chronic renal failure. Chronobiol Int. 1990;7:321-7.
- [Google Scholar]
- Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: An 8-week uncontrolled trial. Am J Kidney Dis. 2007;50:908-17.
- [Google Scholar]
- Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313-21.
- [Google Scholar]
- Effect of valsartan with bedtime dosing on chronic kidney disease patients with nondipping blood pressure pattern. J Clin Hypertens. 2013;15:48-54.
- [Google Scholar]
- Chronotherapy with valsartan/amlodipine fixed combination: Improved blood pressure control of essential hypertension with bedtime dosing. Chronobiol Int. 2010;27:1287-303.
- [Google Scholar]
- Diurnal variation of blood pressure in elderly patients with essential hypertension. J Am Geriatr Soc. 1984;32:896-9.
- [Google Scholar]
- The spectrum of circadian blood pressure changes in type I diabetic patients. J Hypertens. 2001;19:1421-8.
- [Google Scholar]
- The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70:1000-7.
- [Google Scholar]
- Blood pressure 'dipping' and 'non-dipping' in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382-7.
- [Google Scholar]
- High pulse pressure and nondipping circadian blood pressure in patients with coronary artery disease: Relationship to thrombogenesis and endothelial damage/dysfunction. Am J Hypertens. 2005;18:104-15.
- [Google Scholar]







