Translate this page into:
Efficacy of basiliximab induction in poorly matched living donor renal transplantation
Address for correspondence: Dr. V. Sakhuja, Department of Nephrology, Post Graduate Institute of Medical Education and Research, Sector-12, Chandigarh - 160 012, India. E-mail: vsakhuja2009@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Non-depleting antibody induction has the best safety profile in transplant recipients without an increased risk of infection or malignancy. This observational study was performed in intermediate immunologic risk live donor renal transplants to assess basiliximab efficacy in patients on tacrolimus, mycophenolate, and prednisolone immunosuppression. A total of 46 patients on basiliximab induction were compared to risk matched 56 controls at the end of 6 and 12 months post-transplant. An additional cost of approximately Rs. 100,000/patient was incurred by the basiliximab group. The incidence of biopsy proven acute rejection in the control group (12.5%, 6 months and 20.5%, 1 year) and the basiliximab group (13%, 6 months and 18.9%, 1 year) was similar. At 6 months, there was a non-significant trend toward more steroid sensitive rejections and better glomerular filtration rate preservation in the basiliximab group (83.3%, 71.9 ml/min) versus the control group (28.6%, 62.2 ml/min). However, this difference was lost at 1 year (70.1 ml/min vs. 67.6 ml/min). The incidence of infections was similar and none of the patients had a malignancy. Death censored graft survival (94.6% basiliximab and 94.8% control) and the mean number of hospitalizations for all reasons at the end of 1 year were not different among the two groups. In our study, basiliximab induction did not confer an additional advantage in the intermediate risk live donor transplants in patients on tacrolimus and mycophenolate based triple drug immunosuppression.
Keywords
Basiliximab
induction therapy
renal transplantation
Introduction
Renal transplantation is the treatment of choice for patients with end-stage renal disease, to improve both quality of life and life expectancy. The manipulation of the alloimmune response is crucial for successful renal transplantation.
Use of non-depleting antibody induction in live donor kidney transplantation is controversial. In a meta-analysis, the risk of acute rejection was significantly reduced in patients who received interleukin-2 receptor antibody (IL-2R Ab) induction than in those with the placebo at 6 months (12 trials, relative risk 0.66, 95% confidence interval [CI] 0.59-0.74), and at 1 year (10 trials, relative risk 0.67, 95% CI 0.60-0.75).[1] Basiliximab has been proven to be safe and effective in reducing acute rejections in the 1st year after transplantation in trials in which immunosuppression was cyclosporine and azathioprine based.[234] The role of IL-2R Ab in the era of tacrolimus based triple immunosuppression is still being debated particularly in view of the evidence that the effective triple drug immunosuppression negates the benefit of induction.[56] Basiliximab use significantly enhances the cost of immunosuppression. The current study was carried out to analyze the clinical outcomes in terms of graft function, number of rejections, infections, and hospital admissions, in patients who received basiliximab induction with a triple drug regimen of tacrolimus, mycophenolate and steroids, compared to triple immunosuppression alone in Indian population.
Materials and Methods
This was a single center, open label, prospective observational study with follow-up data analysis at 6 months and 1 year. Approval was obtained from the Institute Ethics Committee and informed consent was obtained from all the patients.
All consecutive patients who underwent their first transplant from July 2009 to June 2011 from either spousal, unrelated or poorly matched related donors (defined as less than a haplo match) and receiving triple drug immunosuppression (tacrolimus, mycophenolate, and prednisolone) were included in the analysis. Patients with extended criteria donors, transplantation from a deceased donor and sensitized patients were excluded from the analysis. All patients were offered basiliximab induction therapy. Those patients who opted for the induction regimen constituted the basiliximab group while the rest constituted the control group. All patients received tacrolimus at 0.15 mg/kg/day, mycophenolate 1 g twice daily and 20 mg of prednisolone daily (tapered to 5 mg over the next 8 weeks). Tacrolimus trough levels were targeted at 10-12 ng/ml in the 1st month, followed by 8-10 ng/ml from 2nd to 3rd month and 5-8 ng/ml thereafter in both groups. Tacrolimus levels were monitored on alternate days until discharge and then monthly or as required. Basiliximab was given in a dose of 20 mg IV 2 h before transplantation and on day 4. All patients received trimethoprim-sulfamethoxazole for 6 months while none received valganciclovir prophylaxis. Biopsy was carried out for standard indications and no protocol biopsies were performed.
The primary efficacy end point was the incidence of biopsy proven acute rejection (BPAR) at 6 months and 1 year. Secondary end points included graft dysfunction, response to anti-rejection therapy, infections, and hospitalization episodes and mean glomerular filtration rate (GFR) (Cockroft-Gault formula).
The statistical analysis was performed using SPSS statistics software package version 17.0 developed by IBM corporation. For the demographic data including sex of the recipient and donor and the basic disease Chi-square test was used to calculate the significance. For age, comparison of the means was performed using Student “t”- test. In comparing the deaths, all cause graft dysfunction, etiology of graft dysfunction, rejection episodes, type of rejection and response to therapy and the incidence of various infections, Fischer's exact test or Chi-square test was used to calculate the P value based on the quantity of the variables. The patients who expired during the course of study or lost their grafts were excluded when mean GFR was calculated. A two-tailed P value less than 0.05 was considered significant.
Results
A total of 102 patients were analyzed at the end of 6 months and 76 patients were analyzed at the end of 1 year and the patient disposition is as detailed in Figure 1.
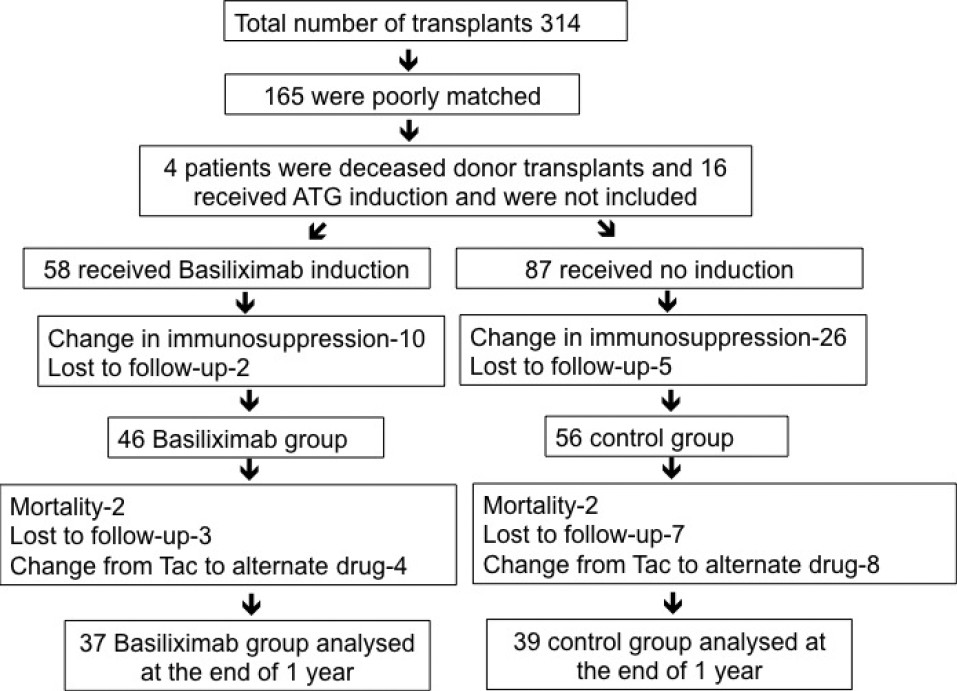
- Flow chart depicting patient enrollment and follow-up
Baseline characteristics
The demographic and baseline characteristics were similar in the two groups; however, the control group was younger (P = 0.01) [Table 1]. In the basiliximab group, each patient incurred an additional expenditure of USD 1500.

Outcomes
The total number of episodes of graft dysfunction and acute rejection were comparable among the groups. Rejection was the most common cause of graft dysfunction followed by infections. The incidence of BPAR was 20.5% in the control group versus 18.9% in the basiliximab group at the end of 1 year [Table 2]. At the end of 6 months, there was a trend toward more steroid responsive rejections in the basiliximab (5/6-83.3%) compared to the control group (2/7-28.6%) (P = 0.1). Late rejections showed poor response in either group with 50% in the control group and 83% in the basiliximab group showing no response to therapy (P = 0.51).

Mean GFR in the basiliximab group was higher (71.94 ml/min) compared to the control group (62.24 ml/min) at the end of 6 months, but this was not statistically significant (P = 0.07). Mean GFR at the end of 1 year was 70.1 ml/min in the basiliximab group and 67.6 ml/min in the control group (P = 0.88).
The incidence of bacterial, mycobacterial, viral, and fungal infections was similar among the two groups. Urinary tract infections were the most common infection among the two groups followed by respiratory infections. One patient had rhinocerebral mucormycosis, cytomegalovirus (CMV) disease occurred in four patients (two in each group) while tuberculosis and BK polyoma virus (BKV) infection was seen in one patient from each group.
None of the patients had a malignancy during the follow-up. Death censored graft survival (94.8% control and 94.6% basiliximab) and the mean number of hospitalizations at the end of 1 year were not different among the two groups (35.8% control and 29.2% basiliximab). There were three deaths in the basiliximab group as compared to two in the control group.
Discussion
The relatively lower costs of induction and side effects with basiliximab compared to anti-thymocyte globulin (ATG) have given significant popularity to basiliximab particularly in low and intermediate risk transplants. However, the cost is still substantial. Introduction of tacrolimus and mycophenolate into the immunosuppressive regimen has improved both short and long term graft outcomes[78] and the efficacy of basiliximab induction in the era of tacrolimus and mycophenolate has not been proven beyond doubt.
Our study population was of the intermediate immunologic risk category. We excluded patients who deviated from the drug protocol (change to alternate immunosuppressive regimen) from further analysis forming a homogenous cohort. However the bias of “differential loss to follow-up” due to economic, geographic reasons, and good graft function might have influenced the overall outcomes.
The incidence of BPAR in the control group (12.5%, 6 months and 20.5%, 1 year) and the basiliximab group (13%, 6 months and 18.9%, 1 year) was similar, but higher as compared to the current United States Renal Data System (USRDS)[9] data of 10%. Follow-up bias might have influenced this outcome. In the largest double-blind placebo controlled randomized study on basiliximab until the date[10] (maintenance regimen are cyclosporine, azathioprine, and steroids), the incidence of acute rejection was 20.8% in the basiliximab group versus 34.9% in the placebo group at 6 months. In the randomized double-blind study by Lawen et al.,[11] the basiliximab group had lesser BPAR episodes compared to the control group (cyclosporine, mycophenolate, and steroid immunosuppression). In the observational study by Cho et al.,[6] where the regimen included tacrolimus, mycophenolate and steroids, basiliximab did not reduce episodes of acute rejection.
Trend toward steroid responsiveness in early rejections as occurred in our study was earlier demonstrated with basiliximab in pediatric transplants.[512] In adults, a similar trend was demonstrated in induction trials by Kahan et al.,[4] where 41.6% in the control group required antibody therapy in addition to steroids as against 25.4% in the basiliximab group. However, the immunosuppression was cyclosporine based in that trial.
Though the better preserved GFR at the end of 6 months approached significance, the advantage could not be maintained at the end of 1 year. Though follow-up bias and chronic rejections could have influenced the ultimate outcome, early preservation of GFR could be due to the steroid responsiveness of acute rejections in the basiliximab group. In the study by Cho et al.,[6] mean creatinine levels at the end of 1 year were not different after basiliximab induction.
Basiliximab did not predispose to increased infections in our study and this is in agreement with previously published data.[23456] Lower incidence of post-transplant malignancies in the sub-continent as reported earlier[13] is further substantiated in our study. Though reported in literature, anaphylaxis with basiliximab[14] seems to be rare as none of our patients had intolerance to basiliximab.
The main limitation of our study is the lack of randomization. Financial issues in the population catered by our hospital hindered a randomized study. The relatively small number of patients and short period of follow-up are other shortcomings. Follow-up bias in view of the geographic and financial reasons and a significant drop out rates could also have influenced the results.
In conclusion, an expensive induction regimen with basiliximab in our study did not confer any significant advantage in intermediate risk live donor transplants on tacrolimus and mycophenolate based triple drug immunosuppression. A larger randomized controlled trial would be useful in defining the role of basiliximab in such recipients.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation. 2004;77:166-76.
- [Google Scholar]
- Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet. 1997;350:1193-8.
- [Google Scholar]
- Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338:161-5.
- [Google Scholar]
- Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation. 1999;67:276-84.
- [Google Scholar]
- A prospective, randomized, multicenter trial of tacrolimus-based therapy with or without basiliximab in pediatric renal transplantation. Am J Transplant. 2006;6:1666-72.
- [Google Scholar]
- Basiliximab does not reduce the early rejection incidence in high-risk kidney recipients under tacrolimus-based immunosuppression. Transplant Proc. 2008;40:2234-6.
- [Google Scholar]
- Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810.
- [Google Scholar]
- Mycophenolate mofetil reduces late renal allograft loss independent of acute rejection. Transplantation. 2000;69:2405-9.
- [Google Scholar]
- USRDS. Transplantation: Outcomes. 2011. ADR. reference tables Available from: http://www.usrds.org/atlas11.aspx
- [Google Scholar]
- A randomized, double-blind trial of basiliximab immunoprophylaxis plus triple therapy in kidney transplant recipients. Transplantation. 2001;72:1261-7.
- [Google Scholar]
- Randomized double-blind study of immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with mycophenolate mofetil-containing triple therapy in renal transplantation. Transplantation. 2003;75:37-43.
- [Google Scholar]
- Open-label, multicenter study on the safety, tolerability, and efficacy of Simulect in pediatric renal transplant recipients receiving triple therapy with cyclosporin, mycophenolate, and corticosteroids. Transplant Proc. 2005;37:672-4.
- [Google Scholar]
- Malignancies following kidney transplantation. J Nephrol Ren Transplant. 2009;2:94-105.
- [Google Scholar]
- Anaphylactic shock caused by immunoglobulin E sensitization after retreatment with the chimeric anti-interleukin-2 receptor monoclonal antibody basiliximab. Transplantation. 2003;76:459-63.
- [Google Scholar]







