Translate this page into:
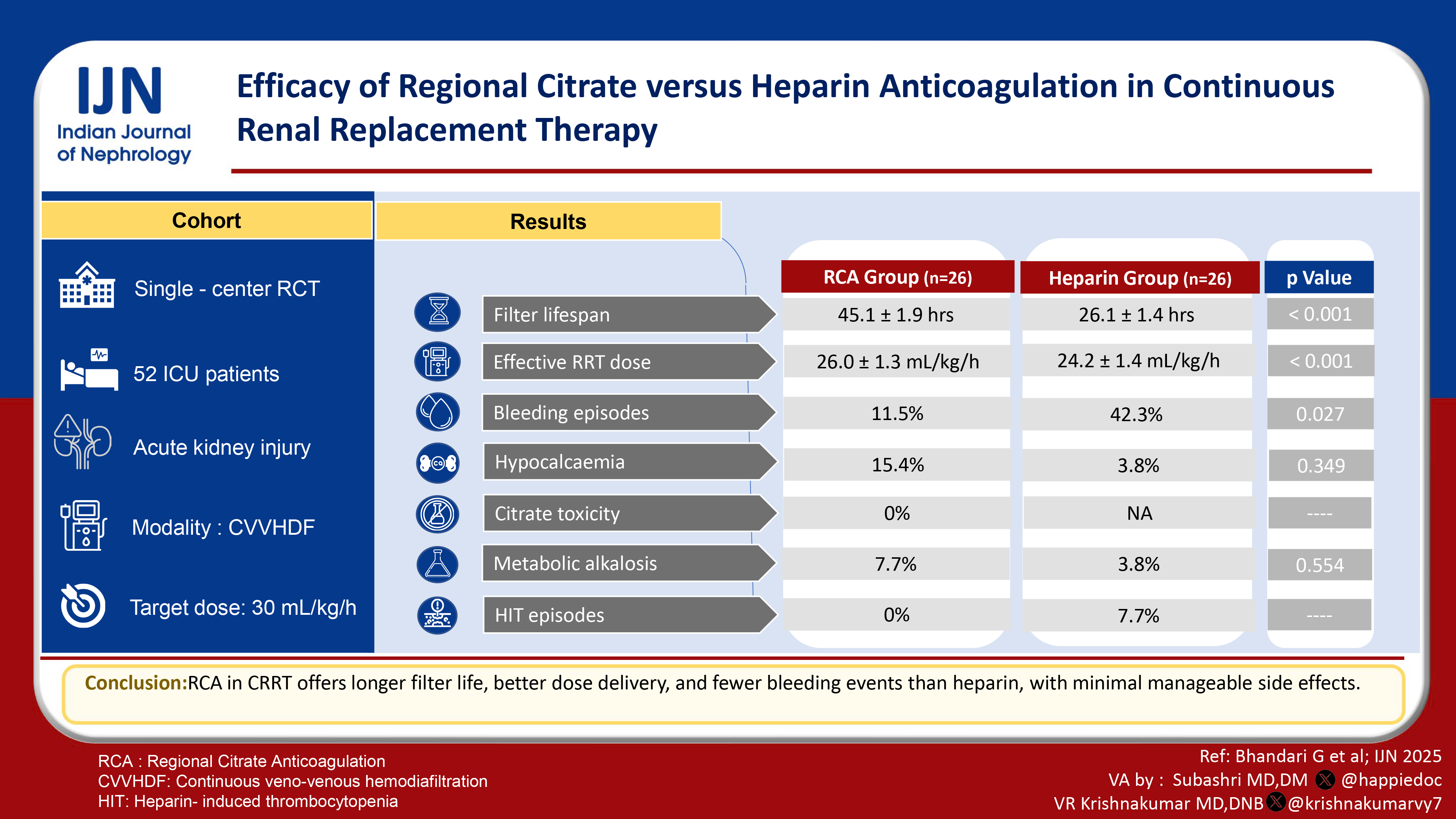
Efficacy of Regional Citrate versus Heparin Anticoagulation in Continuous Renal Replacement Therapy
Corresponding author: Debarun Choudhury, Department of Nephrology, Sir Ganga Ram Hospital, New Delhi, India. E-mail: debc0829@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhandari G, Choudhury D, Bhalla AK, Malik M, Gupta A, Bhargava V, et al. Efficacy of Regional Citrate versus Heparin Anticoagulation in Continuous Renal Replacement Therapy. Indian J Nephrol. 2025;35:380-4. doi: 10.25259/ijn_195_23
Abstract
Background
Continuous renal replacement therapy (CRRT) is used in hemodynamically unstable patients with acute kidney injury (AKI). Heparin, the most commonly used anticoagulant, has a significant bleeding risk and is associated with heparin-induced thrombocytopenia. Regional citrate anticoagulation is an alternative anticoagulation strategy in CRRT.
Materials and Methods
A randomized controlled trial was conducted in the Intensive Care Unit over one year, from October 2020 to September 2021, in patients with AKI requiring CRRT. Fifty-two patients were randomized into two groups: group 1 received regional citrate anticoagulation, and group 2 received heparin anticoagulation.
Results
The mean age in group 1 was 50.46 years, while it was 49.35 years in group 2. The mean filter lifespan in group 1 was 45.11 hours, while in group 2, it was 26.11 hours and was statistically significant (P < 0.001). The mean effective delivered RRT dose was higher in group 1 (26 ml/kg/hour) compared to group 2 (24.23 ml/kg/hour), which was statistically significant (P < 0.001). Bleeding episodes were higher in group 2 than in group 1 (42.3% vs 11.5%), which was statistically significant (P = 0.027). The RCA group had various electrolyte and metabolic complications, but these were not statistically significant.
Conclusion
Regional citrate anticoagulation is better than heparin anticoagulation in terms of filter lifespan, effective delivered RRT dose, bleeding episodes, and metabolic complications.
Keywords
AKI
Anticoagulation
Citrate
CRRT
Introduction
Acute kidney injury (AKI) develops in more than 50% of Intensive Care Unit (ICU) patients. Among ICU patients, mortality is more than 50% in those with AKI, which increases to 80% in those requiring renal replacement therapy (RRT).1-3 Indications to initiate RRT include volume overload resistant to diuretics, refractory severe hyperkalemia with ECG changes, resistant metabolic acidosis, uremic complications like pericarditis, encephalopathy, and bleeding.4 Continuous renal replacement therapy (CRRT) is commonly used modality in patients with hemodynamic instability. The bleeding risk associated with heparin, the most commonly used anticoagulant, ranges from 10% to 50%.5,6 Heparin is also associated with heparin-induced thrombocytopenia (HIT) and faster filter clotting.7,8 Regional citrate anticoagulation (RCA) was first used in the early 1980s. It chelates calcium and inhibits clotting cascade specifically of extracorporeal circulation during CRRT.9 KDIGO suggests the use of RCA as a preferred anticoagulant for CRRT in AKI patients.10
We conducted a randomized controlled trial in AKI patients requiring CRRT. The primary ojective was to compare the efficacy of regional citrate anticoagulation (RCA) versus heparin anticoagulation regarding filter lifespan in patients with AKI on CRRT. Other objectives were to compare the effective delivered renal replacement dose during RCA versus heparin anticoagulation, to assess the safety of RCA in the form of complications like bleeding episodes, hypocalcemia, citrate toxicity, and metabolic complications.
Materials and Methods
This randomized controlled study was conducted in the ICU from October 2020 to September 2021. The required minimum sample size with a significance level of 5% and statistical power of 80% was 26 for each group. Institutional ethical committee approval was taken. Detailed history, clinical examination, and relevant hematological and biochemical investigations were collected. The procedure and complications were explained in detail to them. An informed written consent was obtained before the procedure.
Patients enrolled in the trial were allocated to either RCA or heparin by randomization method. A total of 52 patients were taken and were randomized into two groups. Twenty-six patients received RCA (Group 1), while the remaining 26 received heparin as an anticoagulant for CRRT (Group 2). A randomization list was generated by computer in random blocks of 4 and 8 patients. Sealed, opaque, and sequentially numbered envelopes with the respective allocation cards were prepared. The staff nurse on call would open the envelope each time a patient was enrolled in the study. Blinding was not possible to perform for obvious logistic reasons. A unique identification code was assigned to the subject at inclusion. Data was collected and analyzed using this anonymous number. Flow chart of the study is shown in Figure 1.
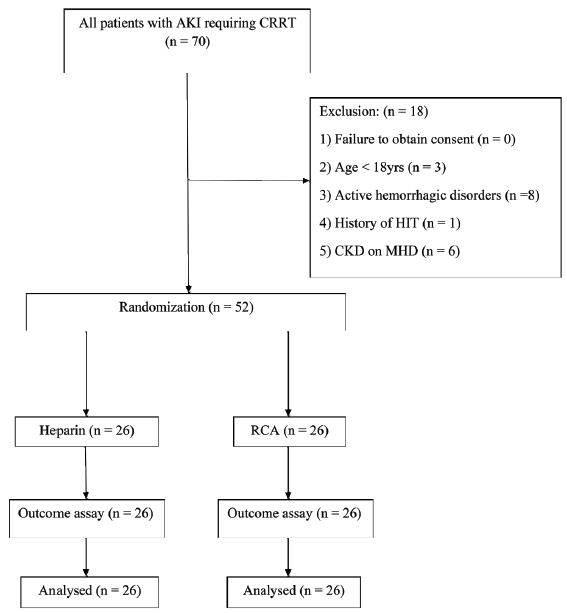
- Flowchart of the study. AKI: Acute kidney injury, CRRT: Continuous renal replacement therapy, HIT: Heparin-induced thrombocytopenia, CKD: Chronic kidney disease, MHD: Maintenance hemodialysis, RCA: Regional citrate anticoagulation.
CRRT was performed using a Prismaflex (Gambro-Dasco, Mirandola, Modena) CRRT machine and a biocompatible high-flux membrane (M100). Continuous veno-venous hemodiafiltration (CVVHDF) was initiated at a dose of 30 ml/kg/h. Replacement fluid was added in both pre- and post-dilution modes (1:1).
The ultrafiltration rate was adjusted according to the patient’s volume status and assessed clinically. Anticoagulation (RCA or heparin) was given in pre-dilution mode. A hemodialysis catheter was inserted into a central vein (femoral or right internal jugular vein). Blood flow was maintained between 50 ml/min and 200 ml/min.
CRRT with citrate as an anticoagulant needed a continuous calcium drip, which was given to the patient in post-dilution mode. The calcium dilution for the drip was 10 ml of 10% calcium chloride in 40 ml of normal saline. Citrate was given in a dose of 3 mmol/L in pre-dilution mode (Regiocit solution was used for RCA, whose citrate concentration is 18 mmol/L). The flow rate of the calcium drip was decided according to the Supplementary Table 1.
Once treatment was initiated and blood flow was established, the following parameters were checked after 60 minutes: ionized Calcium from the patient’s arterial line of hemodialysis catheter and filter Ionized Calcium from the blue port on the CRRT machine.
If the patient’s ionized calcium was less than 0.9 mmol/L at any time during treatment, we administered 10 ml of 10% calcium chloride through a central line over 30 minutes. To prevent filter clotting, a filter-ionized Ca concentration of 0.25-0.35 mmol/L was required. Supplementary Table 2 gives the timings of the filter-ionized calcium and patient-ionized calcium checks.
Adjustments were made through the anticoagulation screen of the CRRT Machine according to Supplementary Table 3.
A high “total calcium to ionized calcium ratio” is a surrogate marker of citrate toxicity. To obtain the value, we performed the following calculation manually:
Patient Total Calcium ÷ Patient Ionized Calcium
After 6 hours of treatment commencing, we did serum total calcium from the biochemistry laboratory. However, increasing calcium compensation in the preceding hours indicated citrate accumulation. A serum total calcium level was checked before the 6-hour mark.
Unfractionated heparin was used as an anticoagulant in pre-dilution mode. Heparin infusion was made by adding 25,000 IU heparin to 50 ml diluent (500 IU/ml). An initial bolus of 80 IU/kg (maximum 5000 IU) was given, followed by a continuous infusion of 10 IU/kg/hr. A minimal maintenance dose of 500 IU/h was used for the patency of the circuit. aPTT was checked 6 hours after the start of the infusion, and the heparin dose was adjusted according to Supplementary Table 4. aPTT was checked 6 hours after each dose adjustment. When in the desired range (46-70 sec), aPTT was monitored daily. The treatment continued until renal function recovery (improved serum creatinine or urine output of ≥1 ml/kg/h) or death. Treatment was discontinued in case of any side effects due to the type of anticoagulation.
Definitions of study outcomes
Filter lifespan:
It was the time duration of use of the CRRT filter in hours from the start of CRRT until there was clotting in the filter, which would be associated with zero flow in the circuit or trans-membrane pressure >250 mmHg, which would be displayed on the CRRT machine.
Effective delivered RRT dose:
It was calculated in ml/kg/h and obtained by dividing the total effective delivered RRT dose (ml/kg) by the total duration of CRRT (hours) when a prescribed RRT dose was given.
Number of episodes of hypocalcemia:
It was measured as ionized calcium from the patient’s sera, and a value of less than 1 mmol/L was considered hypocalcemia.
Number of bleeding episodes from any site (epistaxis, upper GI bleed, etc).
Number of patients in the RCA group with citrate toxicity:
Citrate toxicity was defined as a ratio of total calcium to ionized calcium >2.4, which was calculated manually after measuring total and ionized calcium from the patient’s sera [Supplementary Table 5].
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0. Continuous variables were presented as mean ± SD, and categorical variables were presented as absolute numbers and percentages. The normally distributed continuous variables were compared between the groups using the Student’s t-test. Nominal categorical data between the groups were compared using the Chi-squared test or Fisher’s exact test as appropriate. P < 0.05 was considered statistically significant.
Results
Majority of the patients were male (69.2% in group 1 and 76.9% in group 2). Median age were 50.46 ± 11.03 years in group 1 and 49.35 ± 10.43 years in group 2. Presence of co-morbidities was comparable between the groups [Table 1].
| Characteristic | Group 1 | Group 2 | p value | ||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Females | 8 | 30.8% | 6 | 23.1% | 0.532 |
| Males | 18 | 69.2% | 20 | 76.9% | |
| Diabetes mellitus | 10 | 38.5% | 13 | 50.0% | 0.402 |
| HTN | 14 | 53.8% | 10 | 38.5% | 0.266 |
| CAD | 5 | 19.2% | 4 | 15.4% | 1.000 |
| CAD | 7 | 26.9% | 8 | 30.8% | 0.760 |
| On mechanical ventilation | 14 | 53.8% | 12 | 46.2% | 0.579 |
HTN: Hypertension, CAD: Coronary artery disease, CKD: Chronic kidney disease
Sepsis was the most common cause of hospital admission (38.5% in group 1 and 50% in group 2). Other common indications for hospital admission with AKI requiring RRT in our study included surgery (cardiovascular and abdominal) (30.8% in group 1 and 38.5% in group 2) and trauma (15.4% in group 1 and 7.7% in group 2).
CKD was present in 26.9% of group 1 patients (baseline creatinine- 1.26 ± 0.34 mg/dl) and 30.8% of group 2 patients (baseline creatinine- 1.33 ± 0.36 mg/dl). All of our patients were hypotensive requiring inotropic support with 53.8% in group 1 and 46.2% in group 2 requiring ventilatory support.
Most common indication for kidney replacement therapy was oliguria (53.8% in group 1 and 61.5% in group 2). 5 patients in group 1 and 7 patients in group 2 had severe hyperkalemia (serum potassium>6.5 mEq/L) as an indication of RRT. Two patients in both the groups had resistant volume overload requiring RRT. Five patients in group 1 and 1 patient in group 2 had severe metabolic acidosis requiring RRT. Group 1 had better outcomes as compared to Group 2 [Figures 2-5].
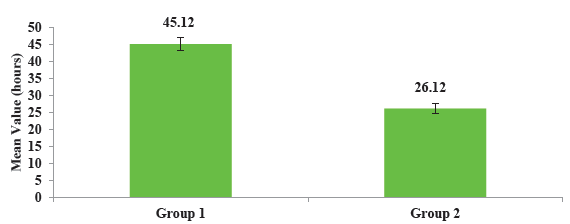
- Comparison of mean filter lifespan between two groups.
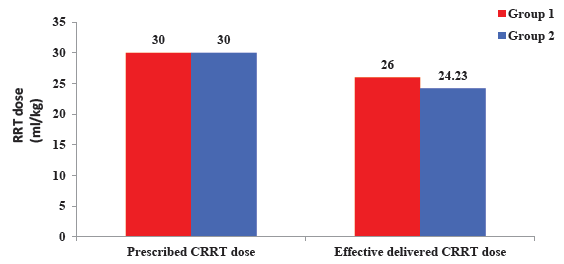
- Comparison of effective delivered RRT dose between two groups. RRT: Renal replacement therapy, CRRT: Continuous renal replacement therapy.
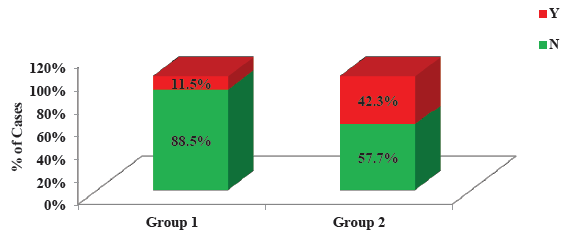
- Comparison of bleeding episodes between two groups.

- Comparison of metabolic complications between two groups.
Filter lifespan was found to be higher in group 1 (45.12 ± 1.92 hours) when compared to group 2 (26.12 ± 1.44 hours) and it was statistically significant (P <0.001).
Daily prescribed RRT dose in both the groups was 30 ml/kg/hour to achieve good hemoperfusion and ultrafiltration. However, effective delivered RRT dose in group 1 (26.00 ± 1.33) was higher than group 2 (24.23 ± 1.42) and was it statistically significant (P <0.001).
In terms of complications, heparin group had higher incidence of bleeding than RCA group (42.3% vs 11.5%) which was statistically significant (P = 0.027). Two patients (7.7%) in heparin group had HIT that led to discontinuation of heparin. Other key findings are summarized in Supplementary Table 6.
Discussion
KDIGO suggests using citrate as an anticoagulant for CRRT.10 However, it is a weak recommendation (Level 2B) with quality of evidence based on small RCTs.11,12 We conducted this trial to compare RCA with heparin anticoagulation in patients with AKI requiring CRRT at our institute.
Sepsis was the most common cause of AKI requiring hospital admission in our study. Other cases of AKI requiring CRRT were post-surgical and trauma cases. This is similar to previous studies on AKI requiring ICU admission or initiation of RRT.13-15
Risk factors for development of AKI include elderly age, co-morbidities like diabetes, hypertension, heart diseases, pre-existing kidney disease, hypotension, drugs like non-steroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers etc.14 In our study, significant proportion of patients had diabetes mellitus, hypertension and coronary artery disease [Table 1].
Indications for initiation of CRRT in our study included oliguria, refractory hyperkalemia, refractory volume overload and severe metabolic acidosis. Similar observations were made by Vaara et al.16
Filter lifespan was found to be higher in group 1 (45.12 ± 1.92 hours) when compared to group 2 (26.12 ± 1.44 hours), and it was statistically significant (P < 0.001). This higher filter lifespan led to decreased filter downtime with a reduced number of filters, thereby decreasing the financial burden of the patients. Our findings of higher filter lifespan with RCA compared to heparin concord with various trials and meta-analyses.11,12,17 A meta-analysis by Bai et al. analyzing 1998 circuits found similar findings (RCA: HR 0.52, P = 0.001 and Heparin: HR 0.76, P = 0.04).18 Another meta-analysis was done by Liu et al., and they found that the mean difference in circuit lifespan between the heparin and RCA groups was 8.18 hours (P < 0.01)5. Similar results were obtained by Monchi et al., Bagshaw et al., and Hetzel et al.11,17,19 These findings of higher circuit lifespan were also observed in children with AKI on CRRT. Fernández et al. conducted a study in children where they found that median circuit survival with citrate and heparin was 48 hours and 31 hours, respectively (P = 0.028), and it was safe with no significant complications.20
The daily prescribed RRT dose in both groups was 30 ml/kg/hour to achieve good hemoperfusion and ultrafiltration. However, the effective delivered RRT dose in group 1 (26.00 ± 1.33) was higher than group 2 (24.23 ± 1.42) and was statistically significant (P < 0.001). Increased filter lifespan leads to decreased circuit downtime and increased delivered RRT dose. Similar findings were found by Stucker et al. where effective delivered RRT doses were 29 ± 5 ml/kg/h and 25 ± 4 ml/kg/h in the RCA and heparin groups, respectively (P = 0.007).9 Thus, our trial showed that RCA is more effective than heparin in filter lifespan and delivered dialysis doses effectively.
In terms of complications, the heparin group had a higher incidence of bleeding than the RCA group (42.3% vs. 11.5%), which was statistically significant (P = 0.027). Liu et al., Monchi et al. and Hetzel et al. also found lower bleeding events with RCA.5,11,19
Heparin-induced thrombocytopenia (HIT) is a known complication of heparin. The incidence of HIT ranges from <1 to 5% depending on the dose and type of heparin.21 Two episodes of HIT were seen in the heparin group. A meta-analysis by Bai et al. also found higher episodes of HIT in the heparin group.18 However, Zarbock et al. did an RCT comparing RCA with heparin in critically ill AKI patients requiring CRRT and found that episodes of HIT were comparable in both groups.22 This can be explained using heparin in the RCA group for DVT prophylaxis.
Regarding safety and complications associated with citrate, four cases of hypocalcemia were seen in the RCA group. However, it was reversed by adjusting the calcium infusion rate and RCA dose. There was one case of hypocalcemia in the heparin group. Two meta-analyses found higher episodes of hypocalcemia in the RCA group but no significant difference in hypocalcemia-related adverse events.5,18
There were zero citrate toxicity cases, defined as the ratio of total calcium to ionized calcium >2.4. This can be explained by the recent citrate protocol for CRRT, which has reduced the incidence of citrate toxicity. Our study found two episodes of metabolic alkalosis with correction of alkalosis by decreasing the RCA dose and stopping bicarbonate infusion if started. Similar findings were found by Brophy et al. where 37 patients with citrate anticoagulation were analyzed. Only 4 cases of metabolic alkalosis and 2 cases of citrate accumulation were seen with reversal by decreasing citrate infusion rate.23 Morgera et al. studied metabolic complications in the RCA group in 209 patients and found that metabolic alkalosis was higher in the RCA group but was correctable by increasing the dialysate flow.24 Various clinical trials have established the safety of RCA when used as an anticoagulant.11,17,23 Our study agrees with previous studies that citrate is a safe and effective anticoagulant compared to heparin. However, robust data on the mortality benefit of citrate over heparin is still not available, and further studies are required.
The limitations of the study includes short duration, small sample size, the study population is limited in diversity as it is a single-center study, no hard endpoints like mortality were evaluated.
Among patients on CRRT, regional citrate anticoagulation, compared to systemic heparin, is effective regarding filter lifespan and delivered RRT dose. It is also safe with few episodes of hypocalcemia and metabolic alkalosis, which are easily corrected by calcium infusion and citrate dose adjustment. No episode of citrate toxicity with a recent RCA prescription has been documented. Heparin was associated with significant bleeding complications and increased heparin-induced thrombocytopenia episodes. RCA is a more effective and safer anticoagulant than heparin in patients with AKI requiring CRRT.
Conflicts of interest
There are no conflicts of interest.
References
- Program to improve care in acute renal disease. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004;66:1613-21.
- [CrossRef] [PubMed] [Google Scholar]
- Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813-8.
- [CrossRef] [PubMed] [Google Scholar]
- Community-acquired acute renal failure. Am J Kidney Dis. 1991;17:191-8.
- [CrossRef] [PubMed] [Google Scholar]
- Renal replacement therapy in the intensive care unit. Ther Clin Risk Manag. 2005;1:141-50.
- [CrossRef] [PubMed] [Google Scholar]
- Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20:1-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Heparin use in continuous renal replacement procedures: The struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol. 1996;7:145-50.
- [CrossRef] [PubMed] [Google Scholar]
- Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: A meta-analysis of randomized controlled trials. Am. J. Kidney Dis. 2012;59:810-8.
- [CrossRef] [PubMed] [Google Scholar]
- Citrate anticoagulation for continuous renal replacement therapy in critically ill patients: Success and limits. Int J Nephrol. 2011;2011:1-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of citrate-based anticoagulation compared to heparin in patients with acute kidney injury requiring continuous renal replacement therapy: A randomized controlled trial. Crit Care. 2015;19:1-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138.
- [Google Scholar]
- Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: A prospective randomized study. Intensive Care Med. 2004;30:260-5.
- [CrossRef] [PubMed] [Google Scholar]
- Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int.. 2005;67(6):2361-7.
- [CrossRef] [PubMed] [Google Scholar]
- Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care.. 2014;18(4):1-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract.. 2013;2013:479730.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal replacement therapy in ICU. J Anaesthesiol Clin Pharmacol.. 2012;28(3):386-96.
- [Google Scholar]
- Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol.. 2014;9(9):1577-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Is regional citrate superior to systemic heparin anticoagulation for continuous renal replacement therapy? A prospective observational study in an adult regional critical care system. J. Crit. Care.. 2005;20(2):155-61.
- [CrossRef] [PubMed] [Google Scholar]
- Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med.. 2015;41(12):2098-110.
- [CrossRef] [PubMed] [Google Scholar]
- Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant.. 2011;26(1):232-9.
- [CrossRef] [PubMed] [Google Scholar]
- Citrate anticoagulation for CRRT in children: comparison with heparin. Biomed Res Int.. 2014;2014:786301.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical review: Anticoagulation for continuous renal replacement therapy-heparin or citrate? Crit Care.. 2011;15(1):1-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA.. 2020;324(16):1629-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT) Nephrol. Dial. Transplant.. 2005;20(7):1416-21.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic complications during regional citrate anticoagulation in continuous venovenous hemodialysis: single-center experience. Nephron Clin Pract.. 2004;97(4):c131-6.
- [CrossRef] [PubMed] [Google Scholar]








