Translate this page into:
Endemic chronic kidney disease of unknown etiology in Sri Lanka: Correlation of pathology with clinical stages
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chronic kidney disease of unknown etiology (CKDU) is endemic among the rural farming communities in several localities in and around the North Central region of Sri Lanka. This is an interstitial type renal disease and typically has an insidious onset and slow progression. This study was conducted to identify the pathological features in the different clinical stages of CKDU. This is a retrospective study of 251 renal biopsies identified to have a primary interstitial disease from regions endemic for CKDU. Pathological features were assessed and graded in relation to the clinical stage. The mean age of those affected by endemic CKDU was 37.3 ± 12.5 years and the male to female ratio was 3.3:1. The predominant feature of stage I disease was mild and moderate interstitial fibrosis; most did not have interstitial inflammation. The typical stage II disease had moderate interstitial fibrosis with or without mild interstitial inflammation. Stage III disease had moderate and severe interstitial fibrosis, moderate interstitial inflammation, tubular atrophy and some glomerulosclerosis. Stage IV disease typically had severe interstitial fibrosis and inflammation, tubular atrophy and glomerulosclerosis. The mean age of patients with stage I disease (27 ± 10.8 years) was significantly lower than those of the other stages. About 79.2%, 55%, 49.1% and 50% in stage I, II, III and IV disease respectively were asymptomatic at the time of biopsy.
Keywords
Endemic chronic kidney disease of unknown etiology in Sri Lanka
interstitial fibrosis
interstitial renal disease
Introduction
Chronic kidney disease of unknown etiology (CKDU) has been recognized to be endemic in several regions, in and around the North Central region within the dry zone of Sri Lanka. The affected regions are, Medawachchiya, Padaviya, Siripura, Horowpathana, Medirigiriya, Polonnaruwa, Nikawewa, Dehiattakandiya and Girandurukotte and more recently recognized regions, Nikawewa and Wilgamuwa[1234] [Figure 1]. Based on persistently elevated urine albumin – creatinine ratio of ≥30 mg/g, kidney diseases have been reported to have a prevalence rate of 15% among the 15–70 year age group in the endemic regions.[5] The population at most risk appears to be the rural male paddy farming community.[46] A recent study has documented higher prevalence of early disease among females, based on persistently elevated urine albumin – creatinine ratio of ≥30 mg/g.[7] However, this observation has not been verified. This disease typically has an insidious onset and a slow downhill progression to end-stage renal disease. Endemic CKDU is the leading cause of indoor mortality in the affected regions and has posed a huge social and economic burden at individual level as well as at national level.

- A map of Sri Lanka showing areas known to be endemic for chronic kidney disease of unknown etiology. The shaded area is the North Central Province
The disease is reported to be more prevalent around ancient water reservoirs that are still utilized by the farmers.[1] Furthermore, the affected villages are clustered along the down streams of the canals draining these reservoirs and the water consumed by the villagers are mostly from shallow wells, ground water tables of which are recharged from the water from these canals.[1] However, the etiology of the disease is still undetermined. Diabetes mellitus and long standing hypertension have been recognized as unlikely associations.[46] Nevertheless, there is an evidence suggesting that the disease is due to exposure to an environmental toxin.[46] Some of the etiological factors under investigation are consumption of pesticide contaminated water,[7] high concentration of fluoride in ground water[28] and other heavy metals such as cadmium and arsenic.[2591011] Ochratoxin A, a well-known nephrotoxin has been identified as an unlikely association.[12]
Endemic CKD have also been reported among the rural farming communities in other parts of the world such as, along the Danube river in the Eastern Europe (Balkan endemic nephropathy),[1314] in Nicaragua,[15] Tunisia[16] and Andhra Pradesh in India.[17] Chronic kidney diseases in Balkan peninsula and Nicaragua have been recognized to be of interstitial type renal diseases.[131415]
In Sri Lankan endemic CKDU, analysis of renal biopsies of asymptomatic patients with CKDU has demonstrated that this too is an interstitial type renal disease and the predominant pathological change in the early disease is interstitial fibrosis.[18] However, the renal pathology in advancing clinical stages of the disease has not been described so far. Therefore, we conducted the following study to correlate the pathological features of the Sri Lankan endemic CKDU with clinical stages of the disease.
Subjects and Methods
This is a retrospective cross sectional study performed on renal biopsies received by the Pathology Department of the Medical Faculty of the University of Peradeniya Sri Lanka, from the Nephrology Units of the Teaching Hospital Kandy and General Hospital Anuradhapura over the period from 2003 to 2007. Renal biopsies from regions known to be endemic for CKDU, namely, Medawachchiya, Siripura, Padaviya, Horowpathana, Medirigiriya, Dehiattakandiya and Girandurukotte were included in the study. A substantial number of patients with asymptomatic CKDU have been shown to have advanced disease.[18] Therefore, cases from symptomatic as well as asymptomatic patients detected by screening programs, which are already being operated, in the high CKDU prevalent areas were included in the study.
The usual screening procedure carried out in these regions is as follows: residents of CKDU prevalent areas were screened for proteinuria with dip stick method in community centers. Those who had proteinuria +1 or above with dip stick method were recognized as “suspected of having CKDU” and referred to local renal clinics, where they were further investigated with proteinuria with dip stick method again, serum creatinine, ultrasound abdomen to assess the renal parenchymal architecture, and fasting blood sugar levels to exclude diabetes mellitus. Those who were recognized or still suspected as having CKDU were referred to the Nephrology Units of Anuradhapura or Kandy Teaching Hospitals for further management and consideration for renal biopsy.
All biopsies were performed as part of the routine diagnostic work-up of the patients. Since this is a high risk population and the pathogenesis and the natural history of the disease are not understood, the threshold for renal biopsy in CKDU endemic regions is lower than those for the nonendemic regions. Therefore, anybody residing in CKDU endemic regions for over 5 years and having any persistent urinary abnormality (tested positive at least on two consecutive occasions), renal insufficiency or abnormal renal parenchymal patterns on ultrasound assessment, in the absence of systemic diseases which can cause renal diseases such as diabetes and hypertension, were suspected as having CKDU and if willing, underwent biopsy. Those who were in end-stage renal disease were not biopsied.
Renal biopsies were collected in 10% formaldehyde for routine processing and fresh for immunofluorescence. Formalin fixed paraffin sections of the renal tissue were stained with hematoxylin and eosin, sliver stains for basement membranes and other special stains when required. Direct immunofluorescence for IgG, IgA, IgM and C3 were performed on fresh samples.
Selection of the study group
Endemic CKDU was defined as a primary interstitial type chronic kidney disease without an identifiable etiology in those living in an area recognized to be endemic for CKDU for a minimum period of 5 years. Accordingly, inclusion criteria for the study group were (1) presence of an interstitial renal disease not secondary to a glomerular disease or systemic disease, (2) absence of specific primary or secondary glomerular disease, and (3) absence of immune deposits as indicated by negative immunofluorescence. Those patients with systemic diseases known to produce renal changes and those who have been living in an endemic region for <5 years were excluded. Accordingly, 251 cases living in an endemic region for over 5 years with histologically proven primary interstitial renal disease were selected as the study group.
Clinical stages
The selected 251 cases were divided into five clinical stages according to clinical practice guidelines of the National Kidney foundation, based on the glomerular filtration rate (GFR) (National Kidney foundation - http://www.kidney.org). Accordingly, the clinical stages were as follows: stage I, GFR >90 ml/min; stage II, GFR 60–89 ml/min; stage III, GFR 30–9 ml/min; stage IV, GFR 15–29 ml/min, and stage V, GFR <15 ml/min. GFR was calculated according to a modification of diet in renal disease formula.
Grading of histological features
Histological features were recorded and graded as follows: Interstitial fibrosis, tubular atrophy and interstitial inflammation were graded on a scale from Mild to severe depicting increasing severity of lesions. The criteria used to grade the severity of each pathological feature are as follows: small patches of interstitial fibrosis involving <10%, multifocal fibrosis involving 10–50% and diffuse or coarse scarring involving >50% of the cortical interstitium were regarded as mild, moderate and severe fibrosis, respectively. Presence of small groups of atrophic tubules involving <10%, multifocal tubular atrophy involving 10–50% and widespread tubular atrophy involving >50% of the cortical tubular sections were categorized as mild, moderate and severe tubular atrophy, respectively. Presence of small collections of lymphocytes and plasma cells involving <10%, multifocal collections involving 10–50% and large collections or diffuse infiltrations involving >50%of the renal cortical interstitium were regarded as mild, moderate and severe inflammation respectively. Numbers of sclerosed glomeruli were given as a percentage of the total number of glomeruli present in the biopsy and was grouped as 0%, 1–50% and >50%. Presence or absence of hypertension associated vascular changes was stated. Hyaline arteriolosclerosis and intimal proliferations of medium size arteries were regarded as hypertension associated vascular changes. Grading of the histological features was performed by two histopathologists independently.
An overall histological grade was given for each case, considering the severity of each histological parameter, using following criteria: overall grade 1 - normal glomeruli without glomerular sclerosis; interstitial fibrosis of any degree, absent or grade 1 interstitial inflammation and tubular atrophy of any degree; overall grade 2 - glomerular sclerosis, interstitial fibrosis of any degree; absent or grade 1 interstitial inflammation and tubular atrophy of any degree; overall grade 3 - with or without glomerular sclerosis, grade 2 or 3 interstitial fibrosis, interstitial inflammation and tubular atrophy; overall grade 4 - advanced glomerular sclerosis, grade 3 interstitial fibrosis and tubular atrophy, interstitial inflammation of any degree-end stage kidney.
Age and the sex of the patients and clinical presentations were retrieved from the archives. Details of the urinary sediment were obtained from the standard urine full reports performed on these patients.
Statistical analysis
Statistical significance of continuous variables was assessed using one way one-way analysis of variance test and the significance of the differences were indentified using Tukey's test. Categorical variables were analyzed comparing proportions. Correlations between GFR and histological gradings were assessed calculating R2 value. Confidence interval was set at 95% for all tests.
Ethical clearance has been granted for this study by the Ethical Review Committee of Faculty of Medicine, University of Peradeniya, Sri Lanka.
Results
Mean age of the cases with endemic CKDU was 37.3 ± 12.5 years, and 193 (76.9%) were males; male to female ratio was 3.3:1. There were 105 (41.8%) cases detected by screening, 44 (17.5%) detected incidentally (e.g. investigating for other illnesses or during routine medical checkups), 91 (36.3%) presented with nonspecific symptoms (e.g. fatigue, back ache etc.) and 11 (4.4%) were diagnosed due to signs and symptoms directly related to renal diseases such as edema. In the screened sample, mean age was 33.4 ± 13.7 years, and 71 (67.6%) were males; male to female ratio was 2.1:1.
Correlation of pathology with clinical stages
There were 77 (30.7%) in stage I disease, 40 (15.9%) in stage II, 108 (43%) in stage III and 26 (24.3%) in stage IV. Patients with stage V disease were not biopsied. Analysis of the screened population revealed that 56 (53.3%) had stage I disease, 10 (9.3%) had stage II disease, 32 (29.9%) had stage III disease and 7 (6.5%) had stage IV disease.
Stage I disease
All had interstitial fibrosis, 50 (64.9%) and 27 (35.1%) had grade 1 and 2 fibrosis respectively and none had grade 3 fibrosis. In 56 (72.7%) interstitial inflammation was absent and 14 (18.2%) and 7 (9.1%) had grade 1 and 2 inflammation respectively; none had grade 3 inflammation. Tubular atrophy was absent in 29 (37.7%) and 36 (46.8%) and 12 (15.6%) had grade 1 and 2 tubular atrophy respectively; none had grade 3 tubular atrophy [Figure 2a and b]. Glomerular sclerosis was absent in 48 (62.3%), 1–50% glomerular sclerosis was present in 29 (37.7%) and none had >50% glomerular sclerosis hypertension associated vascular changes were present only in 1 (1.3%). The mean age of the affected was 27 ± 10.8 years, and there were 50 (64.9%) males and 27 (35.1%) females; male to female ratio was 2:1. 61 (79.2%) patients were asymptomatic, picked up either by screening or incidentally.
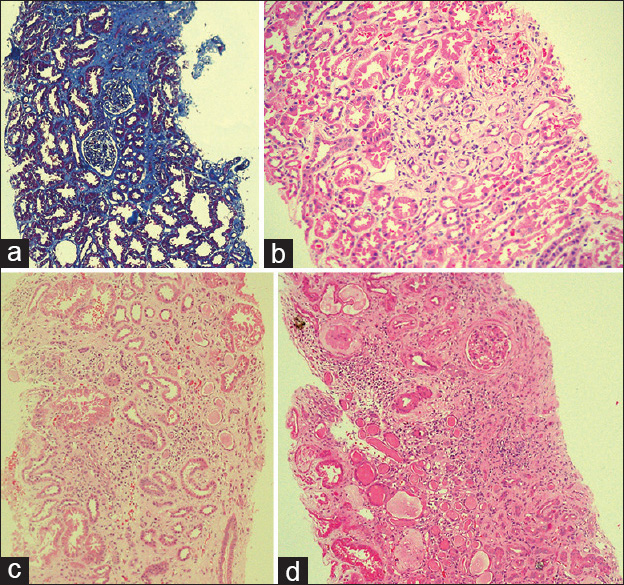
- Pathological features of the endemic chronic kidney disease in Sri Lanka: (a) Stage I disease (Masson trichrome stain), areas of fibrosis stained in blue (10 × 10); (b) stage I disease (H and E): interstitial fibrosis with a few atrophic tubules. There is minimal inflammation (10 × 10); (c) stage III disease: interstitial fibrosis, multifocal tubular atrophy and multifocal infiltrations of mononuclear inflammatory cells (10 × 10), (d) stage IV disease: extensive interstitial fibrosis, tubular atrophy, diffuse mononuclear inflammatory cell infiltration and glomerular sclerosis (10 × 10). (Clinical staging is according to clinical practice guidelines of the National Kidney foundation. The clinical stages are as follows: stage I, glomerular filtration rate (GFR) >90 ml/min; stage II, GFR 60–89 ml/min, stage III, GFR 30–59 ml/min, stage IV, GFR 15–29 ml/min and stage V, GFR <15 ml/min)
Stage II disease
All had interstitial fibrosis and grade 1, 2 and 3 fibrosis were present in 8 (20%), 25 (62.5%) and 7 (17.5%) respectively. Interstitial inflammation was absent in 9 (22.5%) and grade 1, 2, and 3 inflammation were present in 8 (20%), 18 (45%) and 5 (12.5%) respectively. Tubular atrophy was absent in 2 (15%) and grade 1, 2 and 3 tubular atrophy were present in 9 (22.5%), 23 (57.5%) and 6 (15%) respectively. Glomerular sclerosis was absent in 12 (30%), 1–50% glomerular sclerosis was present in 23 (57.5%) and >50% glomerular sclerosis was present in 5 (12.5%). Hypertension associated changes were present in 3 (7.5%). The mean age of the affected was 39.9 ± 13.3 years, and there were 30 (75%) males and 10 (25%) females; male to female ratio was 3:1. 21 (55%) patients were asymptomatic, picked up either by screening or incidentally.
Stage III disease
All had interstitial fibrosis and grade 1, 2 and 3 fibrosis were present in 5 (4.6%), 51 (47.2%) and 52 (48.1%) respectively. Interstitial inflammation was absent in 8 (7.4%) and grade 1, 2, and 3 inflammation were present in 17 (15.7%), 40 (37%) and 43 (39.8%) respectively. Tubular atrophy was absent in 4 (3.7%) and grade 1, 2 and 3 tubular atrophy were present in 6 (5.6%), 49 (45.4%) and 49 (45.4%) respectively [Figure 2c]. Glomerular sclerosis was absent in 12 (11.1%), 69 (63.9%) had 1–50% glomerular sclerosis and 27 (25%) had >50% glomerular sclerosis. Hypertension associated vascular changes were present in 27 (33.3%) cases. The mean age of the affected was 42.4 ± 9.5 years, and there were 92 (85.2%) males and 16 (14.8%) females; male to female ratio was 6:1. 53 (49.1%) patients were asymptomatic, picked up either by screening or incidentally.
Stage IV disease
All had interstitial fibrosis, and there was none with grade I interstitial fibrosis. Grade 2 and grade 3 fibrosis were present in 7 (26.9%) and 19 (73.1%) respectively. Interstitial inflammation was absent in 1 (3.8%) and grade 1, 2 and 3 inflammation were present in 3 (11.5%), 5 (19.2%) and 17 (65.4%) respectively. There was none with absent or grade 1 tubular atrophy. Grade 2 and 3 tubular atrophy were present in 9 (34.6%) and 17 (65.4%) respectively. Glomerular sclerosis was absent in 3 (11.5%), 1–50% glomerular sclerosis in 13 (50%) and >50% in 10 (38.5%) [Figure 2d]. Hypertension associated vascular changes were present in 5 (19.2%) cases. The mean age of the affected was 42.9 ± 8.5 years and there were 21 (80.8%) males and 5 (19.2%) females; male to female ratio was 4:1. 13 (50%) patients were asymptomatic, picked up either by screening or incidentally.
Proteinuria, +1 or more by dipstick analysis, were present in 207 (82.5%). Urine protein quantification results were not available for all patients. Analysis of urine sediment revealed pus cells in two cases in stage I disease, one case in stage II, five cases in stage III and three cases in stage IV. None had pus cell casts. None had significant numbers of red cells in the urine sediment.
The mean age of the stage I disease is significantly lower than the other clinical stages (95% confidence interval using Tukey test for stage I versus II, I versus III and 1 versus IV are 7.56–18.06, 11.31–19.35 and 9.77–22.00 respectively.). The differences of mean ages among stage II, III and IV diseases were not statistically significant [Figure 3].
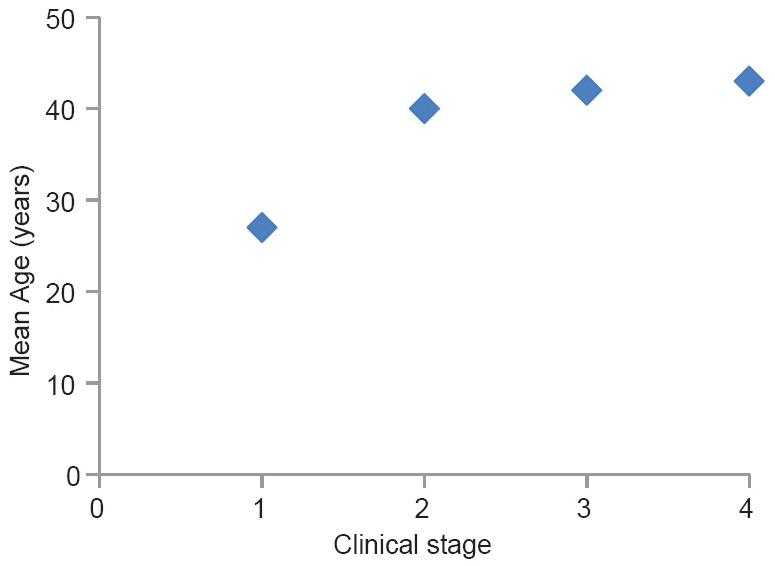
- Mean age against the clinical stage of the disease (clinical staging is according to clinical practice guidelines of the National Kidney foundation)
Males are predominantly affected in all stages. In stage III male predominance is significantly higher compared to stage I (P = 0.002); comparisons between other stages did not show a statistically significant difference.
Correlation coefficient (R2) of each histopathological parameter with mean GFR is as follows: interstitial fibrosis, R2= 0.47 (P < 0.001); interstitial inflammation, R2= 0.47 (P < 0.001); tubular atrophy, R2= 0.5 (P < 0.001); and glomerular sclerosis, R2= 0.33 (P < 0.001). The correlation coefficient of overall histological grade with mean GFR was linear with R2= 0.94 (P < 0.001).
Discussion
Histological analysis, we have conducted on renal biopsies of patients with early endemic CKDU, detected by screening programs, revealed that the overall pathology of asymptomatic CKDU was characterized by consistent presence of interstitial fibrosis with or without interstitial inflammation, tubular atrophy and glomerular sclerosis; viable glomeruli were unremarkable.[18] Criteria adopted for selecting patients for renal biopsy and grading of severity of histological changes in the previous study, and the present study are similar to maintain the comparability of two studies. The overall pathology of the present study sample is similar to those of the asymptomatic population.
In the present study, in stage I and II diseases the predominant pathological change was interstitial fibrosis and in stage I disease 72.2% did not have any inflammation, whereas, prominent concomitant interstitial inflammation was present in stage III and IV disease. According to these observations, it appears that the initial disease is a sclerosing type interstitial disease with no or minimal inflammation and the interstitial inflammation which appears in the more advanced disease stages is probably a secondary manifestation. The other possibility is whether this is a remitting and relapsing type interstitial inflammation, which heals by fibrosis, ultimately giving rise to classic chronic interstitial inflammatory disease as seen in stage III and IV diseases. Since a large majority of patients with the early disease were asymptomatic, we cannot entirely rule out whether the fibrosis in stage I and II disease represents healed foci of interstitial inflammation. It is also possible that both above mentioned variants exist due to different interstitial response patterns to the offending substance. We had similar observations in the previous study involving analysis of asymptomatic individuals.[18] Urine sediment analysis was not helpful in this regard as it did not reveal any significant evidence of ongoing tubular – interstitial inflammation in any clinical stage. A prospective study with close correlation of histological features with clinical evidence of disease activity might provide more information on this.
Once established, interstitial fibrosis plays a crucial role in the progression of renal diseases regardless of the etiology or initial pathological response to the injury. A significant loss of peritubular capillaries has been observed in interstitial fibrosis in animal models and in humans.[1920] Rarefaction of peritubular capillaries and reduction in oxygen diffusion in fibrotic tissue establish chronic local hypoxia which causes tubular atrophy, hypoxic tubular epithelial cell injury and a further fibrosis. Tubular epithelial necrosis induces interstitial inflammation that also leads to a further fibrosis. Obliteration of postglomerular peritubular microvasculature leads to increase in intraglomerular pressure and ischemia, ultimately resulting in glomerular sclerosis.[20] Therefore, regardless of the initial response, with progressive interstitial fibrosis interstitial inflammation, tubular atrophy and glomerular sclerosis set in establishing a vicious cycle and thereby irreversible downhill progression of the disease. The present study demonstrates the progression of the pathological features associated with failing GFR. There is a statistically significant correlation (P < 0.001) between the advancing histopathological parameters and GFR, and the correlation between the overall histological grade, and the mean GFR is linear.
According to the analysis, in general, the typical stage I disease patients are significantly younger than the other stages (mean age 27 ± 10.8 years) and is likely to have mild interstitial fibrosis. Interstitial inflammation and tubular atrophy may or may not be present, and when present they tend to be of mild degree; glomerular sclerosis is usually absent [Figure 2a and b]. The typical stage II disease patient is older (mean age 39.9 ± 13.3 years) and likely to have mild to moderate interstitial fibrosis associated with mild to moderate tubular atrophy; interstitial inflammation may or may not be present and glomerular sclerosis when present tends to be occasional. The typical stage III disease patient is likely to have interstitial fibrosis, interstitial inflammation, tubular atrophy and glomerular sclerosis of moderate severity and the typical stage IV disease patient is likely to have severe interstitial fibrosis and tubular atrophy associated with severe interstitial inflammation and moderate to severe glomerular sclerosis [Figure 2c and d].
Therefore, it is possible that the initial renal injury occurs at a fairly young age represented by residual interstitial sclerosis and the disease progression occurs with ongoing interstitial inflammation and fibrosis due to subsequent repeated exposure to the etiological agent which has been hypothesized to be of environmental origin.[46] Furthermore, the statistically significant age difference between the stage I and the rest [Figure 3] may implicate that the early changes of the disease occur more slowly, that is, stage I disease is more indolent and once the disease process get established the downhill progression becomes relatively rapid. However, further investigation is required to confirm this possibility.
Although, one study, based on persistently elevated urine albumin– creatinine ratio, had reported that females are more affected by the disease,[9] the present study shows that in histologically confirmed disease males are more affected than females in all clinical stages of the disease.
It is also important to note that a significant proportion of patients with endemic CKDU had been asymptomatic in all clinical stages. In stage I particularly, about 80% of the patients had been picked up either by screening programs or as an incidental finding. These observations further highlight the insidious nature of the disease and the need for active surveillance.
Acknowledgment
We are thankful to Mr. G.H.D.S Chandraprabha and Mrs. N. Herath for the technical support provided.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. Ceylon Med J. 2013;58:6-10.
- [Google Scholar]
- Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ Geochem Health. 2011;33:267-78.
- [Google Scholar]
- Prevalence of chronic kidney disease in two tertiary care hospitals: High proportion of cases with uncertain aetiology. Ceylon Med J. 2009;54:23-5.
- [Google Scholar]
- Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011;80:1212-21.
- [Google Scholar]
- World Health Organization Report on Chronic Kidney Disease of Unknown Aetiology in Sri Lanka. Available from: http://www.lankaweb.com/news/items/2012/08/15/world-health-organization- who-report- on-chronic- kidney-disease-of-unknown-etiology-ckdu-in-sri-lanka/
- [Google Scholar]
- Chronic renal failure in North Central Province of Sri Lanka: An environmentally induced disease. Trans R Soc Trop Med Hyg. 2007;101:1013-7.
- [Google Scholar]
- Exposure to acetylcholinesterase-inhibiting pesticides and chronic renal failure. Ceylon Med J. 2006;51:42-3.
- [Google Scholar]
- Dissolution of aluminum from substandard utensils under high fluoride stress: A possible risk factors for chronic renal failures in the North-Central Province. J Nat Sci Found Sri Lanka. 2009;37:219-22.
- [Google Scholar]
- CKDu National Research Project Team. Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180.
- [Google Scholar]
- Chronic kidney disease of unknown aetiology in Sri Lanka: Is cadmium a likely cause? BMC Nephrol. 2011;12:32.
- [Google Scholar]
- Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia) Environ Geochem Health. 2008;30:465-78.
- [Google Scholar]
- Could ochratoxin A in food commodities be the cause of chronic kidney disease in Sri Lanka? Trans R Soc Trop Med Hyg. 2008;102:726-8.
- [Google Scholar]
- Fifty years of Balkan endemic nephropathy: Daunting questions, elusive answers. Kidney Int. 2006;69:644-6.
- [Google Scholar]
- Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798-805.
- [Google Scholar]
- Ochratoxin A and human chronic nephropathy in Tunisia: Is the situation endemic? Hum Exp Toxicol. 2003;22:77-84.
- [Google Scholar]
- Chronic kidney disease in two coastal districts of Andhra Pradesh, India: Role of drinking water. Environ Geochem Health. 2013;35:439-54.
- [Google Scholar]
- Retrospective analysis of renal histology in asymptomatic patients with probable chronic kidney disease of unknown aetiology in Sri Lanka. Ceylon Med J. 2013;58:142-7.
- [Google Scholar]
- Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J Am Soc Nephrol. 2000;11:47-56.
- [Google Scholar]
- Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;19:191-5.
- [Google Scholar]







