Translate this page into:
Etiological Spectrum and Clinical Features in 215 Patients of Crescentic Glomerulonephritis: Is it Different in India?
Address for correspondence: Dr. Vinita Agrawal, Professor, Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow - 226 014, Uttar Pradesh, India. E-mail: vinita@sgpgi.ac.in
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Crescentic glomerulonephritis (CrGN) characterized by the presence of crescents in most (≥50%) glomeruli on renal histology clinically presents as rapidly progressive renal failure. It can occur due to diverse etiologies with varying course and renal outcomes. We studied the prognostic significance of its classification as pauci-immune, anti-GBM, and immune-complex mediated CrGN.
Materials and Methods:
Renal biopsies diagnosed as CrGN over 9 years were included. Clinical, biochemical, serological, and histological features of various classes of CrGN were correlated with renal outcome.
Results:
215 biopsies were diagnosed as CrGN during this period. A majority (63%) were immune-complex mediated while 32% were pauci-immune, followed by anti-GBM disease (5%). 85.5% of pauci-immune CrGN were ANCA associated. The levels of proteinuria and serum creatinine were significantly higher in anti-GBM CrGN as compared to the other two classes. The various histological features including Bowman's capsule rupture, peri-glomerular granulomatous reaction, fibrinoid necrosis, and vasculitis were more common in anti-GBM disease and pauci-immune CrGN. The median renal survival was 6.3, 5.3, 2.1 months in immune-complex mediated, pauci-immune, and anti-GBM CrGN, respectively.
Conclusion:
Immune-complex mediated is the commonest etiology of CrGN in India. Anti-GBM disease has the worst prognosis followed by pauci-immune and immune-complex mediated CrGN. Raised serum creatinine levels (>5mg%) and the degree of glomerulosclerosis at diagnosis were predictors of poor renal survival. High index of suspicion and prompt diagnosis can improve the outcome in these patients.
Keywords
ANCA-associated vasculitis
anti-GBM disease
crescents
glomerulonephritis
Introduction
Rapidly progressive renal failure is a syndrome characterized clinically by rapid and progressive loss of renal function within a few days and weeks. The most common cause on a renal biopsy is crescentic glomerulonephritis (CrGN).[123] CrGN is characterized by the presence of crescents in most (=50%) of the glomeruli. Integration of histology, immunofluorescence, and serology categorizes it into anti-GBM disease, immune-complex mediated, and pauci-immune CrGN.[45]
Anti-glomerular basement membrane (GBM) disease that includes good pasture syndrome and isolated anti-GBM glomerulonephritis is an autoimmune disease characterized by presence of antibodies to the basement membrane proteins with or without systemic involvement. Pauci-immune GN is usually associated with presence of anti-neutrophil cytoplasmic antibodies (ANCA) in the serum. According to clinical features and organ involvement, pauci-immune GN is further classified as granulomatosis with polyangiitis or GPA (formerly known as Wegener's granulomatosis), microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis or EGPA (formerly known as Churg Strauss Syndrome), and renal-limited vasculitis.[67]
Immune-complex mediated CrGN is a manifestation of severe glomerular injury most commonly associated with Lupus nephritis, Henoch-Schönlein purpura, IgA nephropathy, and post-streptococcal glomerulonephritis.[8910]
The prognosis and appropriate management of CrGN varies with the primary cause and the severity of disease at the time of presentation.[3] We have previously shown that anti-GBM CrGN has a poor renal outcome with rapid deterioration requiring renal replacement therapy.[11]
This study was performed to classify CrGN according to etiology as pauci-immune, anti-GBM, and immune-complex mediated CrGN and to evaluate the prognostic significance of this classification. Clinical, biochemical, serological, and histological features of various classes of CrGN at diagnosis were correlated with renal outcomes at follow-up. To our understanding, this is the largest series of CrGN reported from India.
Materials and Methods
The study included 215 renal biopsies diagnosed as CrGN from 215 patients over 9 years, from January 2004 to December 2012, at the Department of Pathology, SGPGIMS, Lucknow. The renal biopsies were performed under local anesthesia by spring-loaded biopsy gun (18G) under ultrasound guidance. Tissue for light microscopy was fixed in 10% buffered formalin. Multiple 3μm thickness serial sections of paraffin-embedded tissue were obtained. All the biopsies were stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), periodic silver methenamine (PSM), and Masson trichrome (MT). Other stains such as Orcein were used as and when required.
Immunofluorescence was performed in all biopsies. Biopsies for immunofluorescence were immersed immediately in isopentane and snap-frozen in liquid nitrogen, embedded in OCT (optimum cutting temperature) medium, sectioned at a 5-μm thickness in a cryostat at -20°C, and stained with fluorescein-conjugated antibodies for IgG, IgM, IgA, C3c, and C1q. The sections were viewed under Nikon Eclipse 80i immunofluorescence microscope. Biopsies with adequate tissue for both light microscopy and immunofluorescence were included in the study. Clinical, biochemical, and serological data were obtained from the medical records. The Institute Ethics Committee approved the study.
The difference between continuous and categorical variables was performed using t-test ANOVA and McNemar's Chi-square tests. Survival analysis was done using Kaplan–Meier survival curves and the difference in renal survival between groups was tested using the log-rank test. Cox regression analysis was performed to analyze the factors predicting time to dialysis dependence in different groups. SPSS (version 15.0) was used for statistical analysis and P value less than 0.05 was considered as significant.
Results
CrGN was diagnosed in 215 (5.7%) of all native biopsies received during the 9-year period. Of the 215 biopsies with CrGN, immune-complex mediated CrGN was the most common (n = 135; 63%) followed by pauci-immune (n = 69; 32%) and anti-GBM disease (n = 11; 5%).
CrGN was more common in males (n = 138; M: F ratio of 1.7:1) and in the 4th –5th decade (range 7–75 years; mean 39.6 ± 15.4 years and median 40 years). The age at onset was higher in patients with anti-GBM (mean 48 ± 15.7 years) and pauci-immune (43 ± 13.8 years) as compared to immune-complex mediated CrGN (mean 37 ± 15.7 years). Immune-complex mediated CrGN was the most common etiology in both pediatric (=18 years) and geriatric (=60 years) patients comprising 82% (14/17) and 58% (14/24), respectively.
CrGN presented with edema (73%), oliguria (50.7%), and gross hematuria (15.8%). Oliguria/anuria was more common (91%) in anti-GBM disease. Extra-renal manifestations in form of arthritis (10.7%) and rash (7%) were more common in immune-complex mediated CrGN. About half (52%) of CrGN were hypertensive at diagnosis. It was more common in anti-GBM disease (n = 7, 63%), followed by immune-complex mediated (n = 71, 53%) and pauci-immune (n = 33, 48%) CrGN.
The biochemical and serological features are shown in Table 1. The mean serum creatinine at the time of diagnosis was 6.5 ± 3.7mg/dl (median 5.7). There was a significant (P = 0.002) difference in serum creatinine levels between the classes, with the highest in anti-GBM disease (10.2 ± 5.3 mg/dl) [Table 1].
| Features | CrGN n=215 | IC-Mediated CrGN n=135 | Pauci-immune CrGN n=69 | Anti-GBM CrGN n=11 | P |
|---|---|---|---|---|---|
| Age (yrs.) Mean±S.D | 39.6±15.4 | 37±15.7 | 43±13.8 | 48±15.7 | 0.008 |
| Age Range (yrs.) | 7-75 | 7-75 | 11-69 | 13-67 | |
| Males:Females | 138: 77 | 90: 45 | 43:26 | 5:6 | 0.345 |
| S. Creatinine (mg/dl) Mean±S.D | 6.4±3.7 | 6.1±3.5 | 6.6±3.5 | 10.2±5.3 | 0.002 |
| 24-hrproteinuria (gm/day) Median (range) | 2.3 (0.12±20.5) | 10.3 (0.17±20.5) | 5.8 (0.12±11.5) | 4.8 (1.1±8.6) | 0.068 |
| Nephrotic proteinuria | 61 (30%) | 46 (34%) | 14 (20%) | 1 (9%) | 0.037 |
| S. Complement C3 (mg/dl) | 96.5±44 | 93.2±45.4 | 96.8±43.7 | 104±41.2 | 0.173 |
| S. Complement C4 (mg/dl) | 29.6±13 | 29.6±13.2 | 30.4±13.2 | 24.8±9.6 | 0.45 |
| Low S. Complement C3 | 35 (16.3%) | 28 (20.7%) | 5 (7.3%) | 2 (18.2%) |
IC-Immune-Complex; Anti-GBM- Anti-glomerular basement membrane
24-h proteinuria varied in different patients with a range of 0.12–20.5 g/day (mean 3.5 ± 3.39, median 2.3 g/day). Nephrotic range proteinuria was significantly more common in immune-complex mediated CrGN as compared to other classes (P value = 0.037). Microscopic hematuria was most commonly seen in anti-GBM disease (82%) followed by immune-complex mediated (77.8%) and pauci-immune (75.3%) CrGN.
The average number of glomeruli in renal biopsies for light microscopy were 15 ± 6 (range 4–40). An average of 63% glomeruli (50–100%) showed crescents, with no significant difference between the classes (P = 0.140). Cellular crescents were more common in immune-complex mediated CrGN (30.8 ± 30%) followed by anti-GBM disease (30 ± 28%). Fibrocellular and fibrous crescents were more commonly seen in pauci-immune CrGN (mean 72 ± 29.7% and 6.7 ± 18.7, respectively). Histological features in the three classes of CrGN are shown in Table 2.
| Features | CrGN n=215 | IC-Mediated CrGN n=135 | Pauci-immune CrGN n=69 | Anti-GBM CrGN n=11 | P |
|---|---|---|---|---|---|
| Glomerular Features | |||||
| Glomerulosclerosis (%) Mean±S.D | 20.4±21.7 | 22±23.5 | 17.5±17.8 | 19.5±21.2 | 0.373 |
| Crescents (%) Mean±S.D | 63.3±20.5 | 65.1±20.5 | 69.2±20 | 76±19.0 | 0.14 |
| Cellular crescents (%) Mean±S.D | 28.8±30 | 30.8±30 | 21.8±30.7 | 30±28.2 | |
| Fibro-cellular crescents (%) Mean±S.D | 68±30.6 | 63.6±30.2 | 72±29.7 | 65±32 | 0.001 |
| Fibrous crescents (%) Mean±S.D | 5.8±19.3 | 5.4±22.9 | 6.7±18.7 | 3±18 | |
| Endocapillary proliferation (n, %) | 147 (68.4%) | 106 (78.5%) | 36 (52.2%) | 5 (45.4%) | |
| Fibrinoid necrosis (n, %) | 57 (26.5%) | 31 (23%) | 20 (29%) | 6 (54.5%) | 0.063 |
| Rupture of bowman’s capsule (n, %) | 98 (45.6%) | 46 (34%) | 45 (65%) | 7 (63.6%) | 0.001 |
| Periglomerular. Granulomatous Inflammation (n, %) | 6 (2.8%) | 0 | 5 (7.2%) | 1 (9%) | 0.005 |
| Tubulo-interstitial Features | |||||
| Tubular atrophy | 203 (94.4%) | 130 (96.3%) | 62 (89.8%) | 11 (100%) | |
| Grade 2 | 42 (19.9%) | 30 (22.7%) | 10 (14.5%) | 2 (20%) | 0.343 |
| Grade 3 | 13 (6.2%) | 3 (2.3%) | 9 (13.0%) | 1 (10%) | |
| Interstitial Fibrosis | 167 (77.7%) | 92 (68.2%) | 45 (65%) | 6 (54.5%) | |
| Grade 2 | 27 (16.2%) | 3 (3.3%) | 2 (4.4%) | 0 | 0.528 |
| Grade 3 | 5 (3%) | 0 | 0 | 0 | |
| Interstitial Inflammation | |||||
| Vascular Features | 211 (98.1%) | 132 (97.8%) | 69 (100%) | 10 (90.9%) | 0.383 |
| Small vessel vasculitis | 4 (1.8%) | 0 | 4 (1.8%) | 0 | 0.000 |
We found endocapillary and mesangial proliferation in 68.4% and 59.5% of renal biopsies of CrGN respectively, with a significant difference between the classes (P = 0.001 and 0.007, respectively). Endocapillary and mesangial hypercellularity were more common in immune-complex mediated CrGN (P < 0.05). Foci of fibrinoid necrosis in glomeruli were more common in anti-GBM disease (54.5%) as compared to immune-complex mediated (23%) and pauci-immune (29%) CrGN. Most of the crescents were fibrocellular (68 ± 30.6%) followed by cellular and fibrous crescents without any significant difference in the proportion of crescents between the classes of CrGN [Figure 1].

- Renal histology showing glomeruli with different types of crescents (a) Fibrocellular and (b) Fibrous crescent (PAS 40X)
Higher incidence of fibrinoid necrosis, hyaline thrombi, and rupture of Bowman's capsule was seen in anti-GBM disease as compared to other classes of CrGN. Rupture of Bowman's capsule was more commonly seen in pauci-immune CrGN as compared to immune-complex mediated CrGN (P = 0.001). Periglomerular granulomatous reaction and inflammatory exudates were significantly higher in pauci-immune CrGN as compared to the other two classes of CrGN. Vasculitis involving interstitial arteries was seen only in 5.8% (4/69) biopsies of pauci-immune CrGN. There was no significant difference in features of chronicity including glomerular sclerosis, tubular atrophy, and interstitial fibrosis between the various classes.
On immunofluorescence, anti-GBM CrGN showed linear glomerular capillary wall staining for IgG, C3c (2-3+ in 64%), IgA (2+ in 18%), and IgM (2+ in 9%). Pauci-immune crescentic glomerulonephritis showed weak (1+) immune deposits in seven and no immune deposits in 62 biopsies. Based on clinical, histological, and immunofluorescence findings, immune-complex CrGN included IgA nephropathy (n = 56), lupus nephritis (n = 25), membranoproliferative glomerulonephritis (MPGN) Type I (n = 21) and MPGN Type II (n = 13), post-infectious GN (n = 8), mesangioproliferative GN (n = 7), membranous nephropathy (n = 2), and others (n = 3). All patients received intravenous methylprednisolone followed by oral prednisolone 0.5 mg/kg for 4 weeks tapered by 2.5 mg/day every 2 weeks and oral Endoxan (1.5 mg/kg).
At the time of presentation, 118 (54.9%) patients were dialysis independent and 97 (45.1%) required dialysis. Follow-up was available in 192 patients with a mean duration of 5.2 months (range 10 days–96 months, median 1.8 months). 21 (9.7%) patients expired within 2–6 months due to septicemia with multi-organ failure and uremia.
At a mean follow-up duration of 170 and 130 days, remission was achieved in only 9% (11/122) and 6.8% (4/59) patients of immune-complex mediated and pauci-immune CrGN, respectively. All the patients of anti-GBM disease were dialysis dependent at follow-up. On correlation of renal outcome with various clinical, biochemical, and histopathological features, higher serum creatinine (>5mg/dl) and degree of glomerulosclerosis at the time of diagnosis were associated with a poor renal outcome (P < 0.05) [Figure 2]. There was no correlation of renal survival with age, gender, presence of hypertension, and the level of proteinuria.
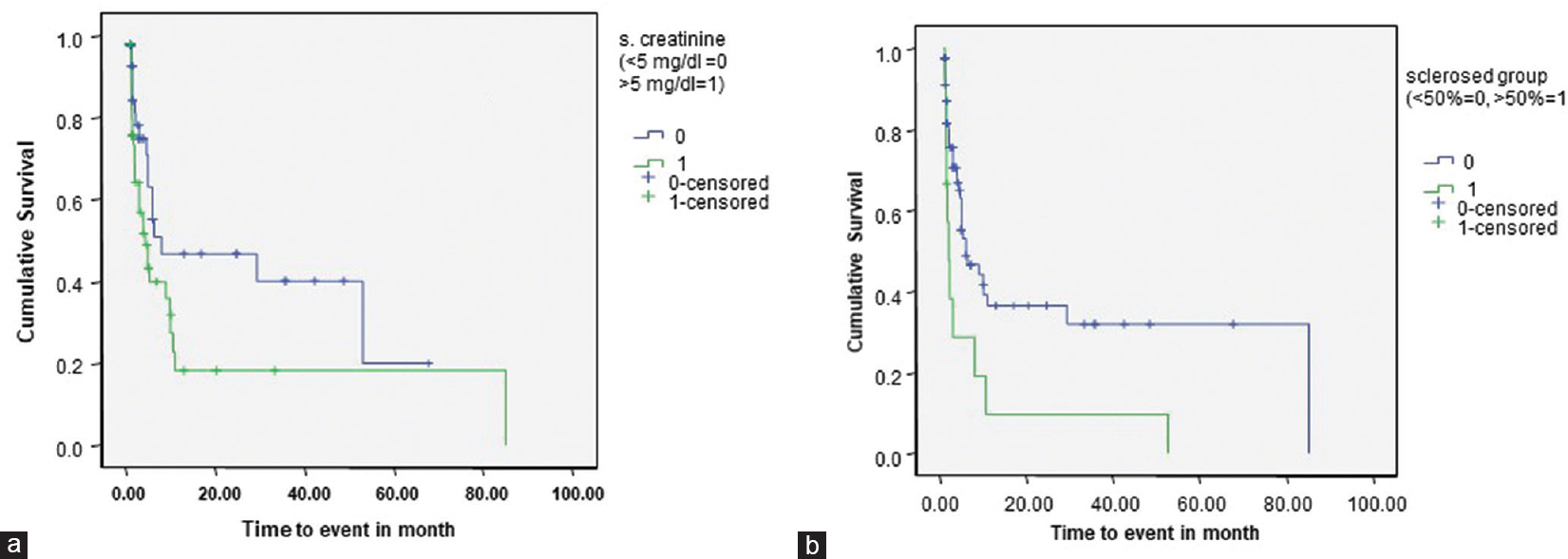
- Poorer cumulative renal survival in patients of Crescentic Glomerulonephritis with (a) Serum Creatinine levels >5 mg/dl (P = 0.048) and (b) presence of >50% sclerosed glomeruli at the time of diagnosis (P = 0.010)
Discussion
We classified CrGN according to etiology as pauci-immune, anti-GBM, and immune-complex mediated CrGN and evaluated the prognostic significance of this classification in our patients. We found immune-complex mediated CrGN to be the most common (135/215, 63%) cause of CrGN, affecting children and adults. This was followed by pauci-immune CrGN 69/215, 32%) and anti-GBM disease (11/215, 5%). The etiologies of immune-mediated CrGN were IgA nephropathy, post-infective glomerulonephritis, membranoproliferative glomerulonephritis, lupus nephritis, and Henoch–Schonlein purpura.
Table 3 summarizes the etiological distribution of CrGN from various geographic regions of the world. In the West, pauci-immune is the most common (~60%) etiology of CrGN followed by immune-complex (~30%) and anti-GBM disease (~10%).[81213] South-East Asia, China, and Macedonia report a relatively higher incidence of immune-complex mediated CrGN.[1415] This has been attributed to the high prevalence of infection of nephritogenic strains in these regions. From China, Chen et al. reported immune-complex CrGN to be the most common (62.7%) followed by pauci-immune (25.8%) and anti-GBM disease (11.6%).[16] This was similar to our findings. In another study of 106 patients of CrGN from North China, Lin W et al. found almost equal incidence of pauci-immune (43%) and immune-complex mediated (41%) CrGN.[17] A study from South India also found immune-complex GN to be the most common etiology (n = 31; 77.5%) followed by pauci-immune glomerulonephritis (n = 8; 20%) and anti-glomerular basement membrane disease (n = 1; 2.5%).[18]
| Authors | Year | Study period | Country | Total | Anti-GBM Disease | Immune-complex mediated CrGN | Pauci-immune CrGN |
|---|---|---|---|---|---|---|---|
| Jennette JC, et al.[8] | 2001 | - | USA | 387 | 95 (24.5%) | 202 (52.2%) | 90 (23.3%) |
| Jennette JC[11] | 2003 | - | USA | 623 | 92 (14.7%) | 154 (24.7%) | 377 (60.6%) |
| Tang Z, et al.[13] | 2003 | 16 years | China | 172 | 15 (8.7%) | 118 (68.6%) | 39 (22.7%) |
| Lin W, et al.[15] | 2010 | - | China | 106 | 17 (16%) | 43 (40.6%) | 46 (43.4%) |
| Suh KS, et al.[35] | 1999 | 7 years | Korea | 17 | 1 (5.8%) | 10 (58.9%) | 6 (35.3%) |
| Rampelli SK, et al.[18] | 2016 | India | 40 | 1 (2.5%) | 31 (77.5%) | 8 (20%) | |
| Chen S, et al.[16] | 2016 | 10 years | China | 528 | 61 (11.6%) | 331 (62.7%) | 136 (25.8%) |
| Present study | 2019 | 9 years | India | 215 | 11 (5.2%) | 135 (62.8%) | 69 (32%) |
CrGN can be an important cause of RPRF in pediatric population with immune-complex mediated CrGN being more common.[3] In a study from the University of North Carolina Nephropathology Laboratory, patients less than 20 years constituted 11.5% of CrGN.[11] We found that CrGN is uncommon in children with only 17 (7.9%) pediatric patients at presentation.
In a large study from Spain, Francisco R, et al. reported 23. 2% of patients of CrGN more than 65 years of age.[19] Moorthy AV, et al. reported 16.5% geriatric patients diagnosed as CrGN on renal biopsy.[20] Jennette JC reported that more than one-third (40.5%) were >60 years of age.[12] In our study, we found only 11% (n = 24) geriatric patients. The age of patients with ANCA GN reported by Jennette JC, et al. (55.86 ± 19.1) was higher than in our patients (43 ± 13.8).[21]
CrGN has been reported more frequently in females as compared to males.[2223] A study from India has shown an almost equal incidence in both genders (male to female ratio 0.9:1) with a significant female preponderance in the immune-complex mediated class.[3] In present study, we found a higher incidence of CrGN in males (64%). However, this could be because of referral bias.
In the present study, the most common extra-renal manifestation was arthritis (n = 23; 10.7%) followed by mucocutaneous manifestations in the form of rash (n = 15; 6.9%) and mucosal ulcers (n = 7; 3.3%). This was comparable to extra-renal manifestations described in other studies.[312]
Most patients of CrGN present with renal insufficiency at the time of presentation manifested by high serum creatinine levels. We found renal insufficiency (high serum creatinine) in most (96.7%) patients of CrGN at the time of presentation. Highest level of serum creatinine was found in anti-GBM disease (10.2 ± 5.3 mg/dl) and lowest in immune-complex mediated CrGN (6.1 ± 3.5mg/dl) and the difference between various classes of CrGN was statistically significant (P = 0.002). Gupta R, et al. Chwee Ang et al., and Sinha A, et al. reported higher mean serum creatinine in pauci-immune CrGN than immune-complex mediated CrGN.[32425]
Morris PA, et al. and Tang Z, et al. have reported nephrotic proteinuria in 28–44% patients of CrGN.[1426] Tang Z, et al. observed highest protein excretion in immune-complex mediated CrGN. We found a 30% incidence of nephrotic proteinuria in CrGN with the highest incidence in immune-complex mediated CrGN (34%) followed by pauci-immune CrGN (20%) and anti-GBM disease (9%).
We observed global glomerulosclerosis in an average of 20% and normal glomeruli in an average of 14.2% biopsies with no statistically significant difference in the proportion of glomerulosclerosis between the classes of CrGN. Similar results are reported in an earlier study from India, where proportion of sclerosed and normal glomeruli in CrGN was 20% and 14.5%, respectively.[25] Heuer, et al. found 29% normal glomeruli and this proportion did not change in follow-up renal biopsies from the same patients, suggesting that disease process affected no glomeruli after therapy was initiated.[27] As described in other studies, we also found no significant difference in the extent of interstitial fibrosis and tubular atrophy between the different etiological groups.[32425]
Jennette JC, et al. have reported immune-complex mediated CrGN to be less aggressive as compared to anti-GBM disease followed by pauci-immune CrGN.[12] In our study, all anti-GBM disease patients developed renal failure. There was no significant difference in survival between pauci-immune and immune-complex mediated CrGN. This could be because most (42%) of our immune-complex, mediated CrGN had IgA nephropathy, which was associated with high incidence of sclerotic lesions.
Severity of renal insufficiency before initiation of treatment is a strong predictor of renal outcome.[23910] Most of our patients had high serum creatinine (>1.3 mg/dl) at the time of diagnosis which could account for the relatively poor renal survival. Pathologic severity in terms of activity of crescents and chronicity of glomerular and tubulo-interstitial disease correlates with prognosis. Degree of glomerulosclerosis and levels of proteinuria, hematuria, and hypertension predict progression to end-stage renal disease.[1228] Presence of fibrous and fibrocellular crescents, tubular atrophy, and interstitial fibrosis at the time of diagnosis are poor predictors of renal survival in CrGN.[2930] We found that the degree of glomerulosclerosis, and serum creatinine >5 mg/dl are associated with a worse outcome.
CrGN requires aggressive prompt management with high-dose corticosteroids and cytotoxic drugs. Plasmapheresis is indicated for anti-GBM disease and ANCA-associated GN with pulmonary hemorrhage.[2910] This study highlights that etiological classification is essential to monitor therapy and predict outcome in of CrGN. Early diagnosis is the key to better renal survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Management of idiopathic crescentic and diffuse proliferative glomerulonephritis; Evidence based recommendation. Kidney Int Suppl. 1999;70:33-40.
- [Google Scholar]
- Diagnosis and management of glomerulonephritis and vasculitis presenting as acute renal failure. Med Clin North Am. 1990;74:893-908.
- [Google Scholar]
- Crescentic glomerulonephritis: A clinical and histomorphological analysis of 46 cases. Indian J Pathol Micro. 2011;54:497-500.
- [Google Scholar]
- Pathology of the Kidney (6th ed). Boston: Jennette JC; Olson JL; Schwartz, Melvin M; Silva FG; 2007.
- Antiglomerular basement membrane antibody-mediated glomerulonephritis and goodpasture's syndrome. Medicine (Baltimore). 1979;58:348-61.
- [Google Scholar]
- Crescentic glomerulonephritis: New aspects of pathogenesis. Semin Nephrol. 2011;31:361-8.
- [Google Scholar]
- ANCA – Associated renal vasculitis -Epidemiology, diagnostics and treatment. Prague Med Rep. 2004;105:237-60.
- [Google Scholar]
- Diagnostic classification of antineutrophil cytoplasmic autoantibody-associated vasculitides. Am J Kidney Dis. 1991;18:184-7.
- [Google Scholar]
- Anti-glomerular basement membrane crescentic glomerulonephritis: A report from India and review of literature. Indian J Nephrol. 2016;26:335-9.
- [Google Scholar]
- Clinical spectrum of diffuse crescentic glomerulonephritis in Chinese patients. Chin Med J (Engl). 2003;116:1737-40.
- [Google Scholar]
- Long-term follow-up of aggressively treated “idiopathic” rapidly progressive glomerulonephritis. Am J Med. 1989;86:400-6.
- [Google Scholar]
- Etiology and outcome of crescentic glomerulonephritis from a single center in China: A 10-year review. Am J Kidney Dis. 2016;67:376-83.
- [Google Scholar]
- The immunopathological spectrum of crescentic glomerulonephritis: A survey of 106 patients in a single Chinese center. Nephron Clin Pract. 2010;116:c65-74.
- [Google Scholar]
- Clinical spectrum and outcomes of crescentic glomerulonephritis: A single center experience. Indian J Nephrol. 2016;26:252-6.
- [Google Scholar]
- Clinicopathologic correlations of renal pathology in Spain. Kidney Int. 2004;66:898-904.
- [Google Scholar]
- Renal disease in the elderly: Clinicopathologic analysis of renal disease in 115 elderly patients. Clin Nephrol. 1980;14:223-9.
- [Google Scholar]
- Idiopathic IgA nephropathy with diffuse crescent formation. Am J Nephrol. 2002;22:480-6.
- [Google Scholar]
- Anti-glomerular basement membrane (GBM)-antibody-mediated disease with normal renal function. Nephrol Dial Transplant. 1998;13:935-9.
- [Google Scholar]
- Etiology and outcome of crescentic glomerulonephritis. Indian Pediatr. 2013;50:283-8.
- [Google Scholar]
- Rapidly progressive glomerulonephritis. A clinical and pathologic study. Am J Med. 1978;65:446-60.
- [Google Scholar]
- Long-term renal injury in ANCA-associated vasculitis: An analysis of 31 patients with follow-up biopsies. Nephrol Dial Transplant. 2002;17:587-96.
- [Google Scholar]
- Prognostic indicators in children with IgA nephropathy report of the Southwest pediatric nephrology study group. Pediatr Nephrol. 1994;8:15-20.
- [Google Scholar]
- Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol. 1991;36:107-13.
- [Google Scholar]
- Anti-GBM disease: Predictive value of clinical, histological and serological data. Clin Nephrol. 1993;40:249-55.
- [Google Scholar]







