Translate this page into:
Hemoglobin Casts in Kidney Biopsies: Etiological Spectrum
Address for correspondence: Dr. Anila A. Kurien, Renopath, Center for Renal and Urological Pathology, No. 27 and 28, VMT Nagar, Kolathur, Chennai - 600 099, Tamil Nadu, India. E-mail: anila_abraham08@yahoo.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Intravascular hemolysis, which is the destruction of red blood cells in circulation, can cause acute kidney injury as the hemoglobin released by the lysed cells is toxic to the tubular epithelial cells. We performed a retrospective analysis of 56 cases of hemoglobin cast nephropathy reported at our institution to analyze the etiological spectrum causing this rare disease. The mean patient age was 41.7 (range: 2–72 years), and the male-to-female ratio was 1.8:1. All patients presented with acute kidney injury. The etiologies include rifampicin-induced, snake bite, autoimmune hemolytic anemia, falciparum malarial infection, leptospiral infection, autoimmune hemolytic anemia, sepsis, non-steroidal anti-inflammatory drugs, ingestion of termite oil, heavy metal poisoning, wasp sting, and valvular heart disease with severe mitral regurgitation. We demonstrate a wide spectrum of conditions associated with hemoglobin casts in the kidney biopsy. Hemoglobin immunostain is required to establish the diagnosis.
Keywords
Acute kidney injury
hemoglobin casts
hemolysis
kidney biopsy
Introduction
Intravascular hemolysis, a relatively rare condition, is the destruction of red blood cells (RBCs) in circulation resulting in the release of free hemoglobin. Hemoglobin is toxic to the tubules and causes acute kidney injury (AKI).[1] There are a variety of causes for hemolysis which are very different between tropical and western countries. Rifampicin-induced and snake envenomation are the major causes of intravascular hemolysis in India.[2] In western countries, autoimmune hemolytic anemia is the major cause of hemoglobin cast nephropathy (HCN).[3] We did a retrospective analysis of 56 cases of HCN. This case series being the largest collection of HCN helps in better understanding the clinicopathological characteristics of this rare disease.
Methods
Medical records of all patients were reviewed from March 2015 to September 2021. Biopsies showing AKI with pigment casts showing hemoglobin positivity were included in the study. There were 56 cases of HCN among 23,434 native kidney biopsies. Clinical history and laboratory findings at the time of performing renal biopsy were recorded.
For light microscopic evaluation, the biopsy samples were fixed in 10% buffered formalin, dehydrated in graded alcohols, and embedded in paraffin. Serial 2-mm-thick sections were cut and stained with hematoxylin and eosin, periodic acid-Schiff, Jones methenamine silver, and Massons trichrome stain. Immunofluorescence study was performed in all cases by using fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgM, IgA, C3, C1q, kappa, and lambda light chains (Dako, Carpinteria, CA). Immunostaining using Cell Marque (Rocklin, CA) antibodies for hemoglobin and myoglobin were done on the biopsies to confirm the diagnosis.
Results
A total of 56 biopsies meeting the inclusion criteria were evaluated. The biopsy prevalence of hemoglobin cast nephropathy was 0.24%. The demographic data for HCN are provided in Table 1. The mean age was 41.7 (range: 2–72 years), and predominantly male patients were affected. Patients in the fourth and sixth decade were slightly more affected than patients in other age groups. The mean serum creatinine was 8.4 mg/dL (1.5–28 mg/dL). All patients presented with AKI. One patient who was on rifampicin had hepato-renal syndrome. Urinalysis revealed variable proteinuria in 43 patients (76.8%) and gross hematuria in 10 patients (17.9%). Comorbid conditions include hypertension in 11 patients (19.6%) and diabetes mellitus in five patients (8.9%).
| Data | Value |
|---|---|
| Age in years: mean (range) | 41.7 (2-72) |
| ≤10 | 3 (5.4%) |
| 11-20 | 3 (5.4%) |
| 21-30 | 8 (14.3%) |
| 31-40 | 14 (25%) |
| 41-50 | 9 (16%) |
| 51-60 | 12 (21.4%) |
| 61-70 | 6 (10.7%) |
| 71-75 | 1 (1.8%) |
| Male/female | 36/20 |
The underlying cause of hemolysis was known in 49 of 56 patients (87.5%). The etiologies include rifampicin-induced, snake bite, falciparum malarial infection, leptospiral infection, autoimmune hemolytic anemia, sepsis, ingestion of toxins (termite oil poisoning and heavy metal poisoning), wasp sting, and valvular heart disease with severe mitral regurgitation [Table 2]. The most common cause was rifampicin-induced and was noted in 17 patients (30.3%). The next common cause was snake envenomation-induced hemolysis and was present in 16 patients (28.4%). Together these two contributed to 58.7% of all the causes.
| Etiology in our study | n (%) |
|---|---|
| Rifampicin | 17 (30.3) |
| Snakebite | 16 (28.4) |
| Autoimmune hemolytic anemia | 4 (7.2) |
| Sepsis | 3 (5.4) |
| NSAID | 2 (3.6) |
| Toxin ingestion | 2 (3.6) |
| Falciparum malaria infection | 2 (3.6) |
| Leptospiral infection | 1 (1.8) |
| Wasp sting | 1 (1.8) |
| Valvular heart disease | 1 (1.8) |
| Unknown | 7 (12.5) |
| Total | 56 |
Acute tubular injury was noted in all patients, with loss of brush border in the proximal convoluted tubules, epithelial simplification, and cytoplasmic vacuolization. Denuded epithelial cells were seen in the lumen of some of the tubules. Hemoglobin casts were seen mostly in the proximal convoluted tubules and less frequently within the distal tubules. The casts were bright red to brown on hematoxylin and eosin stain [Figure 1], weakly periodic acid-Schiff positive, argyrophilic on Jones methenamine silver stain, and weak to brightly fuchsinophilic in Masson trichrome stain. They had a granular to globular texture. No RBC casts were seen in these biopsies. Hemoglobin immunohistochemistry showed granular positivity on the pigment casts [Figure 2]. Faint granular staining was seen within some of the tubular epithelial cells. Two patients of HCN following snake bite also showed positive staining for myoglobin immunostain.

- The pigment casts within the tubules are globular and more reddish-brown as compared to the bright red color of the RBCs in the glomerular capillary tuft. Hematoxylin and eosin stain, ×400
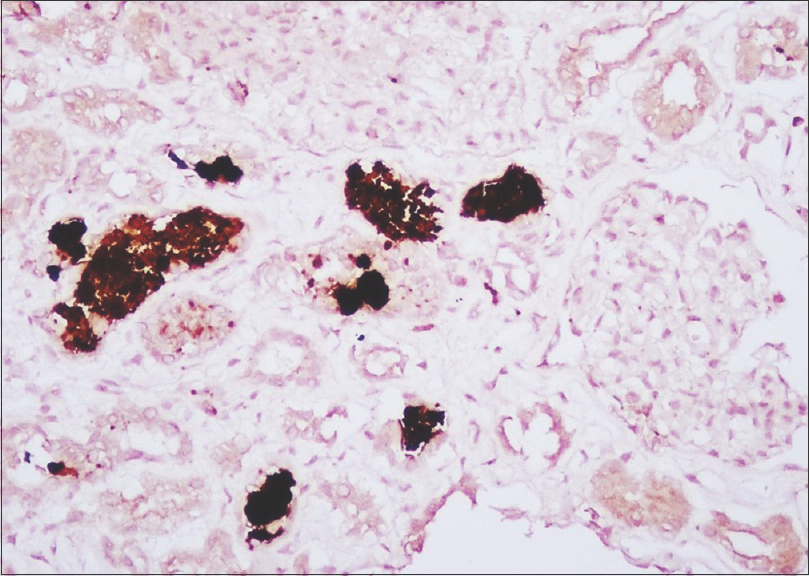
- Intense positivity on the casts for hemoglobin immunostain. Some of the tubular epithelial cells also show positivity as a faint brown blush, ×400
Discussion
The present case series is the largest reported so far, and it expands the spectrum of possible etiologies of this rare clinical condition. In intravascular hemolysis, free hemoglobin is released into the circulation where it dissociates into dimers which bind to haptoglobin.[4] When the binding capacity of haptoglobin is saturated, the free hemoglobin is cleared by the glomerulus. Hemoglobin precipitates in the renal tubules because of its relative insolubility.[4,5] The megalin-cubulin receptor system in the brush border of proximal tubules reabsorbs the hemoglobin. Increased intracellular heme concentration causes cell injury by protein denaturation, lipid oxidation, enzyme impairment, DNA denaturation, and cytoskeletal disintegration.[6,7]
The three major mechanisms by which heme pigment causes kidney injury are[4]
-
Direct injury to tubular epithelial cells
-
Vasoconstriction leading to reduced kidney perfusion
-
Tubular obstruction by casts and cell debris.
The differential diagnoses of hemoglobin casts include myoglobin casts, RBC casts, light chain casts, and cellular debris. Light microscopically, hemoglobin casts are indistinguishable from myoglobin casts. Thus, hemoglobin and myoglobin immunostaining are imperative for correct diagnosis. RBC casts can be identified by the presence of residual red cell morphology. Light chain casts can be identified based on light chain restriction on immunofluorescence study. The absence of staining for hemoglobin and myoglobin helps in identifying cell debris casts.
In our institution, myoglobin and hemoglobin immunostaining are simultaneously done for all biopsies showing acute tubular injury with granular pigmented casts. Rarely, in the same biopsy, both hemoglobin and myoglobin immunostaining can be seen as some etiologies are overlapping (e.g., toxin, sepsis, snake bite, and trauma). Snake venom is known to cause rhabdomyolysis and hemolysis. Two of our patients who developed AKI following a snakebite had pigment casts that were positive for both hemoglobin and myoglobin.
Rifampicin was the most common cause of hemolysis in our study, seen in 30.3% of patients, whereas it accounted for only 14.8% in the study by Dvanajscak et al.[3] Rifampicin, which is routinely used in multidrug treatment regimens for tuberculosis, nontuberculous mycobacterial infections, and leprosy, can cause AKI because of type II or III hypersensitivity reaction. Anti-rifampicin antibodies can develop, which may cross-react with blood group I antigen on RBCs, leading to complement-mediated hemolysis.[8]
Snake envenomation is the second most common cause for HCN in our study, which has not been reported in western countries. Snake venom is composed of different types of proteins and enzymes. Phospholipase A2 in snake venom causes injury to RBCs, platelets, leucocytes, mitochondria, and skeletal muscle fibers.[9] Autoimmune hemolytic anemia was the major cause of intravascular hemolysis in the largest published series from the west by Dvanajscak et al.,[3] seen in 30% of their cases. In our population, it was the cause for HCN in only 7.2% of the cases. Hemolysis occurs during sepsis due to a variety of reasons, including disseminated intravascular coagulation, restriction of glucose to RBCs, changes in RBC membrane properties, pathogens that are hemolytic, and RBC apoptosis.[10]
Nonsteroidal anti-inflammatory drugs (NSAIDs) are known to cause intravascular hemolysis.[11] The drug binds covalently to RBC membrane components and stimulates hapten-dependent antibodies. Diclofenac and ibuprofen were the NSAIDS causing hemolysis in our patients. Malaria causes AKI by hemolysis which can occur early in the course of infection as a result of a high parasite burden or may occur following initiation of anti-malarial treatment.[12] Quinine, which is an antimalarial drug, is known to cause drug-induced immune hemolytic anemia.[11]
Hemolytic anemia associated with leptospirosis is documented.[13] Hemolysis can be due to direct injury to the RBCs by phospholipases of the infecting Leptospira or because of antibody-induced hemolysis. Wasp venom can cause AKI because of intravascular hemolysis, rhabdomyolysis, or shock.[2] The venom contains various biogenic amines, including phospholipases, hyaluronidase, acid phosphatase, histamine, and kinin. Phospholipase A acts on RBC membranes, leading to hemolysis.[14]
While intravascular hemolysis has been frequently reported with mechanical heart valves, hemolysis associated with native valvular heart disease is rare and is most likely due to high shear stress on the RBCs caused by the regurgitation flow.[15] Our patient had severe mitral regurgitation, which may have caused the destruction of RBCs by the collision of the regurgitation flow to the atrial wall.
Conclusion
This study demonstrates a wide spectrum of conditions associated with hemoglobin casts in kidney biopsy. Hemoglobin immunostain is very specific and is required to establish the diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Diagnosis and treatment of autoimmune hemolytic anemia: Classic approach and recent advances. Blood Res. 2016;51:69-71.
- [Google Scholar]
- Clinical profile and outcome of pigment-induced nephropathy. Clin Kidney J. 2018;11:348-52.
- [Google Scholar]
- Hemolysis-associated hemoglobin cast nephropathy results from a range of clinicopathologic disorders. Kidney Int. 2019;96:1400-7.
- [Google Scholar]
- Physiology and pathophysiology of heme:Implications for kidney disease. J-Am Soc Nephrol. 2007;18:414-20.
- [Google Scholar]
- Heme:A-determinant of life and death in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F370-7.
- [Google Scholar]
- Intracellular targets in heme protein-induced renal injury. Kidney Int. 1998;53:100-11.
- [Google Scholar]
- Acute renal failure due to rifampicin: A-study of 25-patients. Am J Kidney Dis. 2002;40:690-6.
- [Google Scholar]
- Clinicopathological spectrum of snake bite-induced acute kidney injury from India. World J Nephrol. 2017;6:150-61.
- [Google Scholar]
- One center's experience: The serology and drugs associated with drug-induced immune hemolytic anemia--a new paradigm. Transfusion. 2007;47:697-702.
- [Google Scholar]
- Acute renal failure following multiple wasp stings. Nephrol Dial Transplant. 1999;14:214-7.
- [Google Scholar]
- Intravascular hemolysis in patients with mitral regurgitation: Evaluation by erythrocyte creatine. J-Cardiol. 2018;71:414-8.
- [Google Scholar]







