Translate this page into:
Etiological Spectrum and Histopathological Diagnosis of Rhabdomyolysis Associated Myoglobin Cast Nephropathy in South India
Address for correspondence: Dr. Anila Abraham Kurien, Renopath, Center for Renal and Urological Pathology, No 27 and 28, VMT Nagar, Kolathur, Chennai - 600 099, Tamil Nadu, India. E-mail: anila_abraham08@yahoo.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Aims:
Rhabdomyolysis occurs due to injury to skeletal muscle fibers and the release of muscle constituents into the circulation. Myoglobin cast nephropathy leading to acute kidney injury is one of the most severe complications of rhabdomyolysis. This is a retrospective study which aims to analyse the clinicopathological features of myoglobin cast nephropathy.
Methods:
A total of 57 cases of myoglobin cast nephropathy were identified after performing immunohistochemical staining for myoglobin on all renal biopsies with pigment casts. The clinical, laboratory data, histopathological findings and clinical outcome of these cases were evaluated.
Results:
The mean patient age was 34.47 years (range 17-77) and the male to female ratio was 6.1:1. All patients presented with acute kidney injury with mean serum creatinine of 8.4 mg/dl (range: 1.7 to 20.8 mg/dl). Rhabdomyolysis was clinically suspected only in 31 patients. Along with myoglobin casts, acute tubular injury was present in all the biopsies. The most frequent conditions associated with myoglobin cast nephropathy in our study were snake envenomation and unaccustomed physical activities. A few activities that caused rhabdomyolysis in our patients were unique to India.
Conclusion:
Clinicians should be aware of the wide spectrum of causes for rhabdomyolysis. The classical clinical and laboratory findings of rhabdomyolysis may not be present in many of the patients. The pathologist must have a high index of suspicion, and immunohistochemical stain should be used to confirm the diagnosis.
Keywords
Immunohistochemistry
myoglobin
myoglobin cast nephropathy
rhabdomyolysis
Introduction
Rhabdomyolysis is a biochemical and clinical syndrome occurring due to necrosis of skeletal muscle fibers and leakage of muscle contents into the circulation. Myoglobin is filtered by the glomeruli and form tubular casts.[1] There are diverse causes for rhabdomyolysis, with a great variation between western and tropical countries. In western countries, exogenous toxins like illicit drugs, alcohol and prescribed drugs are the most common causes. This is followed by muscle diseases, trauma, seizures, immobility and metabolic causes.[12] The major causes of rhabdomyolysis in India include snake envenomation, wasp sting, strenuous exercise and seizures.[3] We sought to retrospectively analyse the clinicopathological characteristics of myoglobin cast nephropathy in a large series of renal biopsies.
Methods
Medical records from January 1, 2015 to October 31, 2019 were reviewed. We receive renal biopsies from multiple institutions in south India. Clinical history and laboratory findings at the time of performing the renal biopsy were recorded. For light microscopy, the biopsy samples were fixed in 10% buffered formalin, dehydrated in graded alcohols and embedded in paraffin blocks. Serial 3-micron sections were cut and stained with hematoxylin and eosin, Periodic acid–Schiff (PAS), Jones methenamine silver and Massons trichrome stain for all biopsies. Immunofluorescence study was performed in all cases using fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgM, IgA, C3, C1q, kappa and lambda light chains (Dako, Carpinteria, CA). Pigment casts were identified in 116 patients. Immunohistochemistry to confirm the presence of myoglobin casts was done on these cases using rabbit polyclonal anti-myoglobin antibody (Cell Marque, Rocklin, CA). Among the 116 patients, myoglobin immunostain was positive over the casts in 57 patients (49.1%). Only those biopsies with positive myoglobin immunohistochemistry are included in this study. Patients with clinical suspicion of rhabdomyolysis but without IHC proven myoglobin casts were not included in this study.
Results
The demographic data is listed in Table 1. The highest incidence was seen in the 20-40 years' age group. Except for hypertension in 5 and diabetes in 2 patients of age >40 years, these were no other known co-morbid conditions. All patients presented with AKI. The mean serum creatinine at the time of performing biopsy was 8.4 mg/dl (range 1.7 to 20.8 mg/dl). Rhabdomyolysis was clinically suspected only in 31 (54.4%) patients.
| Data | Value |
|---|---|
| Age in years: mean (range) | 34.47 (17-77) |
| ≤20 | 7 (12%) |
| 21-40 | 29 (51%) |
| 41-60 | 16 (28%) |
| ≥60 | 5 (9%) |
| Male/female | 49/8 |
Creatinine phosphokinase (CPK) was performed at the time of biopsy only in 28 (49.1%) patients. It was >1500 IU/L in 19 (67.9%) patients and was <1500 IU/L in the remaining 9 (32.1%) patients. The mean value of serum CPK was 2410 IU/L (range: 600 to 26660 IU/L).
A vast variety of etiologies led to rhabdomyolysis with AKI and myoglobin cast nephropathy in our patients, with snake envenomation and unaccustomed physical exertion being the most common causes. The causes in our patients are very different from that in the western world.[2] The various etiologies are listed in Table 2.
| Causes in our study | n (%) | Causes in the study by Liapis et al.[2] | n (%) |
|---|---|---|---|
| Snake envenomation | 12 (21.1) | Drug administration (polydrug abuse, cocaine, heroin, opium, opioids) | 26 (12.2) |
| Exertional rhabdomyolysis | 11 (19.2) | Obtunded (falling, traffic injury, found unconscious, etc) | 15 (7) |
| Seizures | 7 (12.2) | Dehydration | 14 (6.5) |
| Wasp sting | 5 (8.7) | Infection | 13 (6.1) |
| Drug induced | 4 (7) | Seizures | 10 (4.7) |
| Rat killer poison | 3 (5.2) | HIV patients | 10 (4.7) |
| Alcohol abuse | 3 (5.2) | Sepsis | 9 (4.2) |
| Hyperpyrexia | 2 (3.5) | Pancreatitis | 5 (2.3) |
| Sepsis | 2 (3.5) | Chemotherapy | 4 (1.9) |
| Scrub typhus | 1 (1.8) | Myopathies | 3 (1.4) |
| Myositis | 1 (1.8) | Intense physical activity (marathon runners) | 3 (1.4) |
| Multiple substance abuse | 1 (1.8) | Post surgery | 3 (1.4) |
| Prolonged immobile state | 1 (1.8) | Malignant hypertension | 2 (0.9) |
| Unconscious patient | 1 (1.8) | Wasp stings | 2 (0.9) |
| Dehydration | 1 (1.8) | Multiorgan failure | 2 (0.9) |
| Unknown | 2 (3.5) | Transplantation | 12 (5.6) |
| Unknown | 81 (37.9) | ||
| 57 | Total | 214 |
In all patients, renal biopsy revealed acute tubular injury. Many of the tubular epithelial cells were swollen. There was cytoplasmic vacuolation and attenuation of the tubular epithelial cells. Some of the epithelial cells had sloughed off. On the hematoxylin- and eosin-stained sections, the casts appeared pink to reddish brown [Figure 1]. They were granular to globular, some had a ropy appearance [Figure 2]. The casts appeared weakly PAS positive and brown to black on Jones methenamine silver stain and fuchsinophilic on Massons trichrome stain [Figure 3]. They gave positive immunohistochemical staining for myoglobin [Figure 4]. There was mild interstitial edema in some of the biopsies. No significant inflammatory infiltrate was identified. None of the biopsies revealed significant glomerulosclerosis, interstitial fibrosis or tubular atrophy. Three patients had co-existing IgA nephropathy.

- Granular casts with tubular epithelial injury (hematoxylin and eosin; original magnification, X 200)
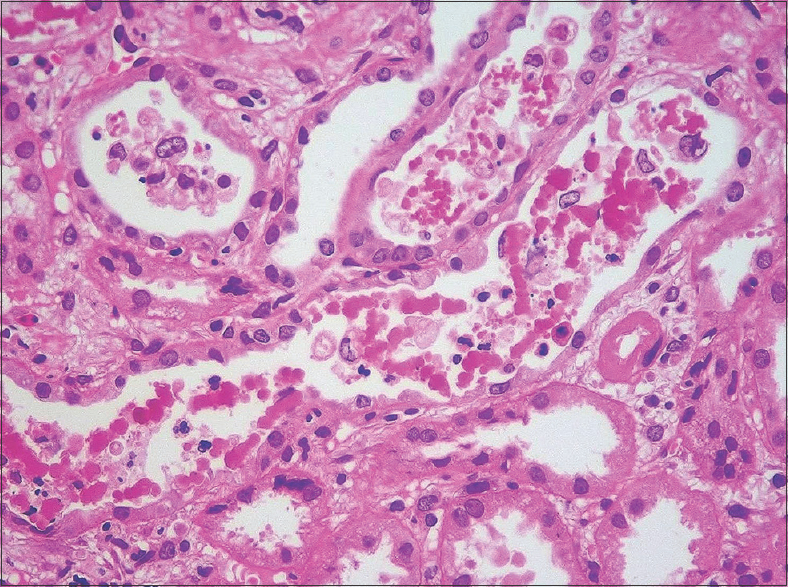
- Some of the casts have globular to ropy appearance (hematoxylin and eosin; original magnification, X 400)
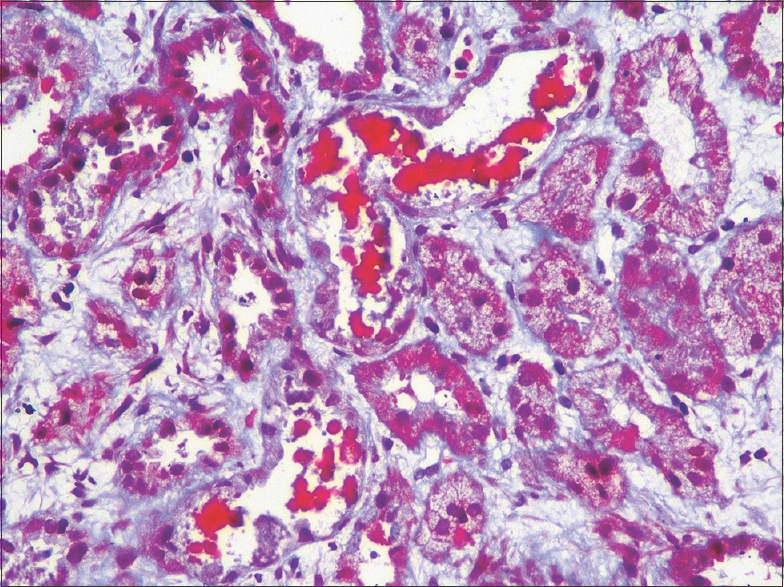
- Fuchsinophilic globular casts (Masson trichrome stain; original magnification, X 200)
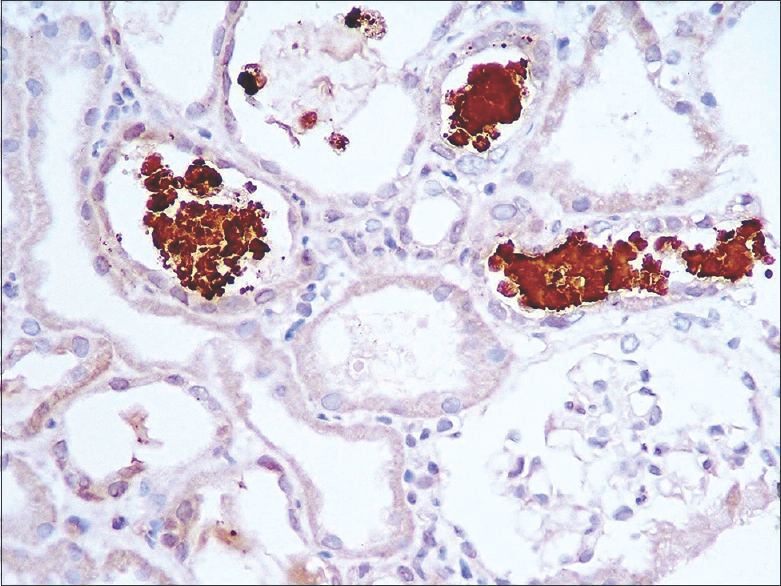
- Casts are strongly positive with anti-myoglobin antibody (Cell Marque, Rocklin, CA, original magnification, X 200)
Information on the treatment provided was available for 46 patients. Forty-one of them underwent hemodialysis. Follow-up details were available for 31 patients. Renal function improved in all but 4 patients.
Discussion
Rhabdomyolysis refers to rapid breakdown of striated muscles resulting in the release of myoglobin, muscle enzymes including CPK, calcium, potassium and other intracellular constituents into the extracellular space and circulation.[4] Myoglobin is a heme protein which contains iron in its ferrous (Fe+2) form. The renal threshold for myoglobin is 0.5 -1.5 mg/dl above which it appears in the urine.[3] When a large amount of myoglobin enters the lumen of the renal tubule it precipitates with Tamm-Horsfall protein to form myoglobin casts. This process is favoured by acidic urine. Also, reactive oxygen species which are generated during damage to muscle cells oxidises the ferrous iron in myoglobin to ferric oxide (Fe+3), generating hydroxyl radical in the process. Both the heme moieties and the hydroxyl radicals cause direct toxicity, mainly to the proximal tubular epithelial cells.[5] The 3 principal mechanisms underlying kidney injury caused by myoglobin include renal hypo-perfusion/vasoconstriction, cast formation, and direct cytotoxicity.[6].
Myoglobin casts are usually granular to globular, they appear eosinophilic to brown in hematoxylin and eosin stain, weakly PAS positive and bright red in Masson trichrome stain.[2] Not all patients with rhabdomyolysis have myoglobin casts, but all patients with myoglobin casts have rhabdomyolysis.[4] Myoglobin casts have to be differentiated from other pigment casts like haemoglobin casts and bile casts. Haemoglobin casts which are seen in cases of intravascular haemolysis are morphologically indistinguishable from myoglobin casts on routine light microscopy.[7] Rhabdomyolysis is the most common cause of pigment nephropathy and is followed by haemolysis.[3] Myoglobin immunostain is very specific and does not cross react with haemoglobin or any other casts.[27] Immunostain for haemoglobin helps to identify haemoglobin casts.[7] Sometimes, cellular debris from acute tubular injury may resemble granular type of myoglobin casts.[2] Bile casts are greenish brown and they are positive for Hall's stain.[8].
The etiology of rhabdomyolysis varied widely among our patients. Snake venom is a complex poison composed of hundreds of proteins like enzymes and polypeptide proteins. Of these phospholipase A2 causes skeletal muscle injury.[9] Twelve of our patients had snake bite induced AKI with myoglobin cast nephropathy. Previous studies have also observed that snake envenomation is the leading cause of rhabdomyolysis in India.[3].
Among the 57 patients, 11 patients had history of unaccustomed strenuous physical excretion. Rolling prostration, known as 'sayana or anga pradakshinam' in Sanskrit, is a ritual where the devotee rolls around the santum santorum of the temple in the lying position keeping both hands above the head and palms joined together. One patient presented with AKI after this ritual. Two patients developed AKI after performing 'girivalam', a ritual practised in Tamil Nadu where they walked barefoot for 14 kilometres around the Arunachala Temple. Five patients developed myoglobin cast nephropathy following prolonged workout at the gymnasium. The other causes of strenuous activity in our patients include a case each of near drowning, scuba diving and wrestling. Exertional rhabdomyolysis is much more common than traumatic rhabdomyolysis and is referred to as 'white collar rhabdomyolysis', due to its significantly higher incidence observed in educated and professionals who are not accustomed to strenuous exercise.[10] Unaccustomed physical exercise in hot and humid conditions can lead to rhabdomyolysis. The continuous muscle contractions compromise capillary blood flow.[11] In a large case series of persons with near drowning in the Mediterranean Sea, features of rhabdomyolysis were detected in most patients due to muscle hypoxia and the strenuous physical exercise in their struggle to stay alive.[12].
Seven patients had seizures before the onset of renal failure. A seizure episode can cause rhabdomyolysis due to the extreme muscular activity, resulting in a state in which ATP production cannot keep up with the demand, subsequently exhausting cellular energy supplies leading to a disruption of muscle cell membranes.[13].
Wasp sting usually causes local allergic reactions. Phospholipase and mellitin in wasp venom can lead onto rhabdomyolysis and haemolysis.[14] The pigment casts have to be differentiated by immunohistochemical stains. Five of our patients developed myoglobin cast nephropathy following wasp sting. One of our patients developed myoglobin cast nephropathy following scrub typhus infection. Scrub typhus caused by Orientia tsutsugamushi contributes to a significant number of community acquired AKI in tropical countries due to hypotension, vasculitis, septic shock and multiorgan dysfunction. Scrub typhus producing rhabdomyolysis is very rare with only a few case reports.[15].
The other etiologies for rhabdomyolysis in our patients included drugs (4 patients), consumption of rat killer poison (3), alcohol abuse (3), hyperpyrexia (2), sepsis (2), multiple substance abuse (2), myositis (1), prolonged immobile state (1), dehydration (1), and unknown (2).
Muscle pain, weakness and dark coloured urine are the classic triad of rhabdomyolysis.[1] This was not observed in many of our patients. Serum CPK is a sensitive marker for muscle injury. There is an acute increase in the serum CPK concentration to more than 5 times the normal upper limit (after excluding myocardial infarction, CKMB fraction should be less than 5%) following muscle injury. Serum CPK levels increase within 12 hours of onset of muscle injury and reaches baseline in 3 to 5 days.[16] The mean CPK level in our study was 3340 IU/L. The variation in CPK levels in our patients could be because CPK levels returned to normal by the time the patient developed AKI. Also, it has been suggested that CPK levels in traumatic rhabdomyolysis correlated better with predicting the development of AKI than CPK levels in non-traumatic AKI.[17].
The metabolism of myoglobin is rapid and unpredictable. The half-life of circulating myoglobin is only 2-6 hours. Serum myoglobin returns to normal level within eight hours. When urine myoglobin concentration is more than 250 μgm/ml (normal <5 ng/ml), visible myoglobinuria occurs (cola or tea-coloured urine). For myoglobin dipstick to be positive, urine myoglobin concentration should be atleast 60 μg/ml.[1819] The measurement of urine or serum myoglobin has a low sensitivity.[20] By orthotoludine dipstick, myoglobinuria was positive only in 42% of our patients. At the time of biopsy, the diagnosis of rhabdomyolysis was made in only 54.4% of our patients. Hence, the renal pathologist must have a high index of suspicion and IHC should be performed if pigment casts are seen in the biopsy.
Prognosis of myoglobinuric AKI is usually good.[21] Despite the fact that our patients presented with AKI with high creatinine levels that required haemodialysis, the renal function in most of our patients returned to normal. In comparison, in the study from USA, only 45% of the patients in whom follow up details was available had full recovery. 18% of the patients in the study died, possibly due to underlying disease. The better prognosis in our patients could be because the majority of them were young, previously healthy individuals without associated co-morbidities.
Conclusion
Rhabdomyolysis-associated myoglobin cast nephropathy results from a wide variety of clinicopathological conditions. Clinicians should be aware of the wide spectrum of causes, some of which are unique to India. The pathologist must have a high index of suspicion and immunohistochemical stain should be used to confirm the diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Rhabdomyolysis: An evaluation of 475 hospitalized patients. Medicine. 2005;84:377-85.
- [Google Scholar]
- Myoglobin casts in renal biopsies: Immunohistochemistry and morphologic spectrum. Hum Pathol. 2016;54:25-30.
- [Google Scholar]
- Clinical profile and outcome of pigment-induced nephropathy. Clin Kidney J. 2018;11:348-52.
- [Google Scholar]
- Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314-26.
- [Google Scholar]
- Hemolysis-associated hemoglobin cast nephropathy rsults from a range of clinicopathologic disorders. Kidney Int. 2019;96:1400-7.
- [Google Scholar]
- Bile cast nephropathy: A pathologic finding with manifold causes displayed in an adult with alcoholic steatohepatitis and in a child with wilson's disease. Case Rep Nephrol Dial. 2018;8:207-15.
- [Google Scholar]
- Clinicopathological spectrum of snake bite-induced acute kidney injury from India. World J Nephrol. 2017;6:150-61.
- [Google Scholar]
- Catastrophic medical events with exhaustive exercise: “White collar rhabdomyolysis.” Kidney Int. . 1990;38:709-19.
- [Google Scholar]
- White collar rhabdomyolysis with acute kidney injury. Indian J Nephrol. 2016;1(26):449-51.
- [Google Scholar]
- Rhabdomyolysis following status epilepticus with hyperuricemia. Medicine. 2018;97:e11281.
- [Google Scholar]
- Scrub typhus associated acute kidney injury—A study from a tertiary care hospital from western Himalayan state of India. Ren Fail. 2013;35:1338-43.
- [Google Scholar]
- Rhabdomyolysis: Historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-56.
- [Google Scholar]
- Thevalue of serum creatine kinase in predicting the risk of rhabdomyolysis induced acute kideny injury: A systematic review and meta-analysis. Clin Exp Nephrol. 2016;20:153-61.
- [Google Scholar]
- Ultrafiltration discrepancies in recovery of myoglobin from urine. Clin Chem. 1996;42:965-9.
- [Google Scholar]
- Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: Implications for follow-up. Crit Care Med. 2002;30:2212-5.
- [Google Scholar]
- The syndrome of rhabdomyolysis: Complications and treatment. Eur J Intern Med. 2008;19:568-74.
- [Google Scholar]







