Translate this page into:
Evaluation of Factors Influencing Outcomes in Pauci-Immune Crescentic Glomerulonephritis: Single Centre Experience of 51 Cases
Address for correspondence: Dr. Pallav Gupta, PDCC Renal Pathology, Consultant Pathology, Sir Ganga Ram Hospital, New Delhi - 110 060, India. E-mail: pallavkmc1@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Pauci-immune crescentic glomerulonephritis (PICGN) is rare form of glomerulonephritis that frequently presents as rapidly progressive renal failure. Several prior studies have evaluated role of various factors influencing outcomes in patients with PICGN. The histopathological classification proposed by Berden a decade earlier described difference in the outcomes of patients in the focal, crescentic, mixed and sclerotic category with best prognosis for focal and worst for sclerotic group. The newly proposed renal risk score of Brix takes into account both the histopathological parameters (% of normal glomeruli, tubular atrophy and interstitial fibrosis) and clinical parameter (eGFR) which influences outcome.
Methods:
Retrospective study was performed between 2014 to 2018. Biochemical parameters and ANCA details were recorded and renal histopathology slides were reviewed and classified according to Berden's histopathologic classes. All the cases were further characterized into three groups based on renal risk score (Brix et al). Univariate, multivariate analysis for risk factors predicting ESRD and Kaplan Meier Survival Analysis were done.
Results:
In the present study, we found eGFR (P 0.024), % of normal glomeruli (P 0.023) and IFTA (P 0.001) as important factors influencing renal outcome in patients with PICGN. More than 60% patients achieved complete remission with low renal risk score as compared to patients with high renal risk score in which 80% patients developed ESRD or death at follow up. We also found significant difference in survival among various renal risk categories (Log-Rank P = 0.001) as compared to Berden's international histological classification (Log-Rank P = 0.037) on Kaplan –Meier survival analysis.
Conclusion:
PICGN is a significant cause of mortality and morbidity. Renal histological factors such as % normal glomeruli at time of biopsy, degree of IFTA and renal risk score play an important role in assessing prognosis in these patients.
Keywords
Crescentic
glomerulonephritis
outcomes
Pauci-immune
renal failure
Introduction
Pauci-immune crescentic glomerulonephritis (PICGN) is an important cause of rapidly progressive glomerulonephritis (RPGN) and can accounts for 80% of such cases. Morphologically it is characterized by necrotizing and crescentic glomerulonephritis with minimal to no glomerular staining for immunoglobulin or complement on Immunofluorescence microscopy.[123] Histological classification proposed by international working group a decade earlier described prognosis of PICGN cases based on the four categories, namely focal, crescentic, mixed, and sclerotic. Several studies have validated this as useful prognostic classification,[456] but other studies with multivariate analysis have not found it useful in predicting outcome in cases with PICGN.[789] Recently, a renal risk score was proposed with three risk categories (low, medium and high) in patients with PICGN based on % normal glomeruli, interstitial fibrosis and tubular atrophy (IFTA) and eGFR.[10] Our study evaluated the international histological classification and renal risk scores along with other factors for predicting outcome in patients with PICGN.
Methods
A retrospective observational study was done for cases diagnosed as PICGN from January 2014 to December 2018. The biochemical parameters including serum creatinine, protein-creatinine ratio (PCR), urine examination findings, ANCA status (IIF and ELISA) were obtained from hospital records. Renal biopsy slides stained with Hematoxylin and Eosin stain, Periodic acid Schiff, Periodic acid silver methanamine and Masson's trichrome stain were reviewed for glomerular changes including type of crescent, % normal glomeruli, globally sclerosed glomeruli, Rupture of Bowman's capsule. Tubulo-interstitial compartment was evaluated for inflammation and fibrosis and blood vessel were evaluated for any evidence of vasculitis.
Statistical analysis
The data was analyzed using SPSS 17.0.0 software. Continuous variables were reported as mean and standard deviation and categorical variables were reported as percentage. Univariate analysis and multivariate Cox regression analysis was done to determine the risk factors predicting outcome. Kaplan Meier survival analysis was also done. A P value of <0.05 was considered to be statistically significant.
Results
A total of 64 cases were diagnosed as PICGN during the study period. Of these, 3 pediatric patients were excluded. Fifty nine of the 61 adult patients had adequate number of glomeruli in biopsy (>7 glomeruli) but of these eight patients had inadequate follow-up. Therefore, a total of 51 adult patients were included in the study. Mean age of these patients was 51.2 ± 15.7 years with male to female ratio of 1.2:1. The mean serum creatinine at the time of biopsy was 7.05 ± 4.57 mg/dl and the mean eGFR was 13.6 ± 12.16 ml/min/1.73m2. There were 33 ANCA positive (65%) [Table 1]. Eighty percent patients had proteinuria at time of biopsy of which 9 had nephrotic range proteinuria. Renal biopsies of the study population were characterized into focal (n = 4), crescentic (n = 29), mixed (n = 12) and sclerotic (n = 6) groups according to international histological classification (Berden's) for PICGN. One of the representative cases of crescentic category is depicted in Figure 1a and b. On histology, granulomas were present in 3, vasculitis in 1, fibrinoid necrosis of glomerular tuft in 10 and breach of Bowman's capsule in 11 patients. Majority of the study subjects (29/51, 57% patients) had predominance of cellular crescents at time of biopsy. Study subjects were also characterized into low renal risk (group I), medium renal risk (group II) and high renal risk (group III) categories as per the renal risk score (sum of the total scores of % of normal glomeruli N0:<10% (score 0), N1:10-25% (score 4), N2:>25% (score 6); tubular atrophy and interstitial fibrosis t1:≤25% (score 0), t2:>25% (score 2) and eGFR, G0:>15 ml/min (score 0), G1:≤15 ml/min (score 3). There were 8 cases in renal risk group I, 23 cases in renal risk group II and 20 cases in renal risk group III. Univariate analysis was done for factors including age, eGFR, % of normal glomeruli, interstitial fibrosis and tubular atrophy (IFTA), histologic classes and renal risk score categories [Table 2]. eGFR at biopsy (P 0.024), % IFTA (P = 0.001), % normal glomeruli in biopsy (P 0.023) were significant baseline risk factors to predict ESRD. In multivariate Cox regression analysis considering age, eGFR at time of biopsy, IFTA and % normal glomeruli, both IFTA (<0.001) and % normal glomeruli (P = 0.018) emerged as important predictors of ESRD [Table 3]. Kaplan-Meier survival analysis was done for histological classes and renal risk score categories. There was a significant difference in survival between low, medium and high renal risk categories (P = 0.001, Log rank (Mentel-Cox), Figure 2., survival analysis for various Berden's histologic classes (P = 0.037, Log rank (Mentel-Cox) is depicted in Figure 3.
| Parameter | Value |
|---|---|
| Age | 51.2 yr |
| Gender | |
| Male | 28 |
| Female | 23 |
| Serum creatinine at biopsy | 7.05±4.57 mg/dl |
| eGFR at biopsy | 13.6±12.16 ml/min/1.73 m2 |
| ANCA positiveC-ANCAP-ANCA | 331815 |
| ANCA negative | 18 |
| Proteinuria at time of biopsy | |
| Present | 41 |
| Absent | 10 |
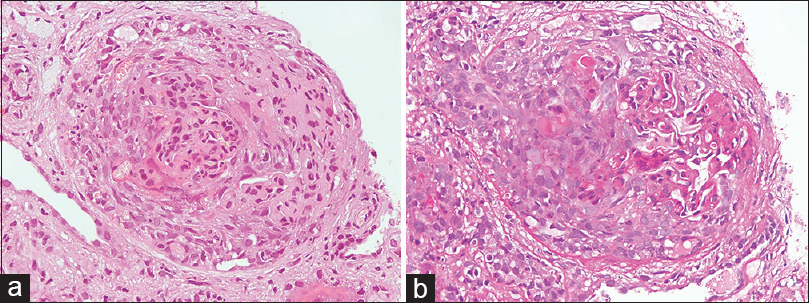
- (a). Section showing a glomerulus with a cellular crescent (Hematoxylin and Eosin, original magnification ×400). (b). Section showing a glomerulus with a cellular crescent and breach of bowman's capsule (Periodic acid Schiff stain, original magnification ×400)
| Parameter | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.006 | 0.979-1.034 | 0.665 |
| eGFR | 0.923 | 0.861-0.990 | 0.024 |
| IF/TA | 4.883 | 1.935-12.325 | 0.001 |
| % normal glomeruli | 0.962 | 0.931-0.995 | 0.023 |
| Berden’s class I | Reference | ||
| Berden Class II | 0.000 | 0.000-2.026 | 0.951 |
| Berden Class III | 12.871 | 0.000-2.939 | 0.957 |
| Berden Class IV | 12.323 | 0.000-2.816 | 0.958 |
| Renal risk group I | Reference | ||
| Renal risk group II | 2.253 | 0.000-3.079 | 0.900 |
| Renal risk group III | 5.714 | 0.000-7.803 | 0.891 |
| Parameter | HR | CI | P |
|---|---|---|---|
| Age | 1.023 | 0.995-1.052 | 0.107 |
| eGFR | 0.949 | 0.872-1.032 | 0.218 |
| IF/TA | 7.08 | 2.665-18.811 | 0.000 |
| % normal glomeruli | 0.956 | 0.922-0.992 | 0.018 |
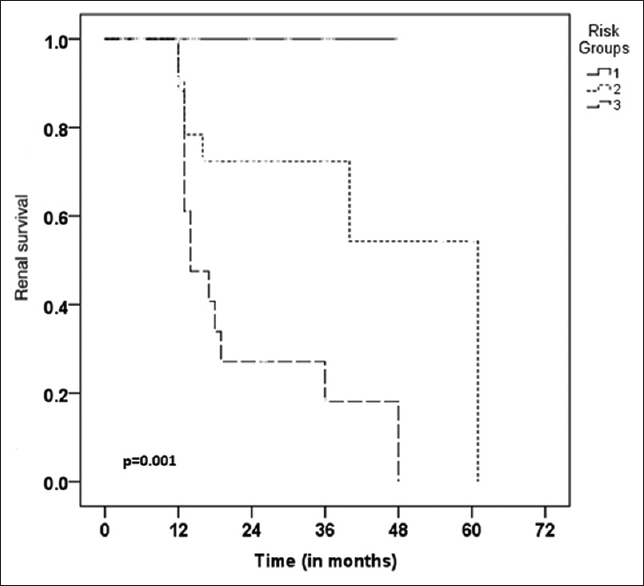
- Kaplan-Meier survival analysis for renal risk categories, low (risk score 1), medium (risk score 2), high (risk score 3)
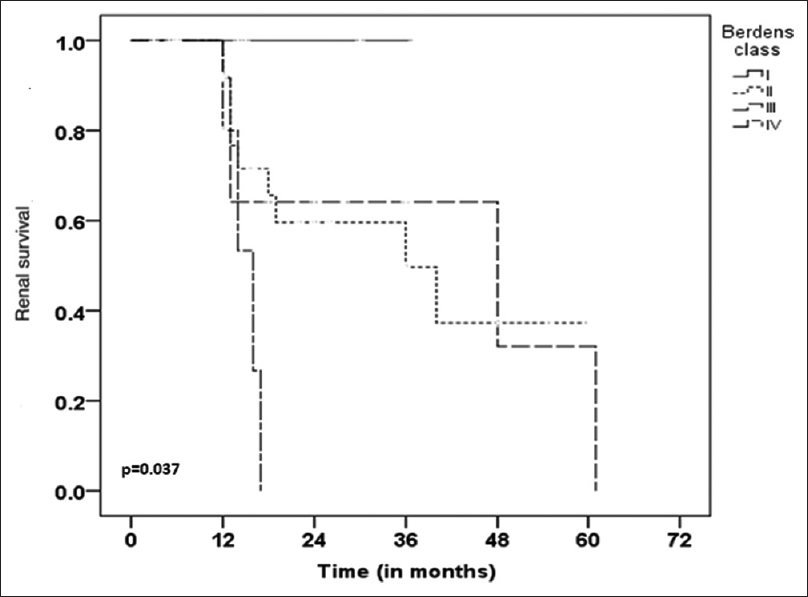
- Kaplan-Meier survival analysis for Berden's histological categories
Intravenous methyl prednisolone was used in 29 patients and plasmapheresis was used in 5 patients. Cyclophosphamide was used as an inducing agent in 45 patients (15 mg/kg every 2 weeks, 3 doses and then every 3 weeks, 7 doses) with azathioprine in maintenance phase. Oral steroids (1 mg/kg) were given for 2 weeks and gradually tapered thereafter. Eighteen patients required hemodialysis.
The follow up period of the patients after biopsy ranged from 12 months to 61 months with a mean follow up of 21 months. In our study, we found that 62.5% of the patients in renal risk category I (low) achieved remission on treatment with no incidence of death or ESRD whereas in renal risk category 2 (medium), 44% patients developed ESRD or deaths with only 22% patients achieving remission. Renal risk category III (high) had the worst outcome with 80% patients developing ESRD or death on follow up with only 10% patients achieving remission [Table 4].
| Deaths | ESRD | Refractory | Remission | |
|---|---|---|---|---|
| Renal risk group I (8) | 0 | 0 | 3 | 5 |
| Renal risk group II (23) | 3 | 7 | 8 | 5 |
| Renal risk group III (20) | 3 | 13 | 2 | 2 |
Discussion
PICGN is more common in whites as compared to blacks.[2] It is a severe form of glomerulonephritis and in untreated patients one-year mortality can be as high as 80%. Renal manifestations of PICGN are severe and progression to end stage renal disease can be prevented by timely diagnosis and initiation of appropriate immunosuppressive regimen. Gold standard treatment for patients with PICGN comprises of corticosteroids (intravenous methylprednisolone followed by oral steroids) and cyclophosphamide.[11] Studies have shown that with immunosuppressive therapy, 5 year survival can be as high as 75% although it can increase morbidity in these patients.[1213] Our study shows that 35% patients with PICGN can be ANCA negative on serology. Similar results have been documented by another Indian study in which 38% patients were ANCA negative.[14] In another study from china 33% cases of PICGN were ANCA negative.[15]
Earlier studies have shown other factors including entry serum creatinine, arteriosclerosis in renal biopsy, higher degree of proteinuria at biopsy as predictors of ESRD.[16] Renal histological classification proposed by Berden and developed by international working group in 2010 showed benefits in terms of predicting long term outcomes in patients with PICGN. It was a purely histological classification and several multivariate analysis have found it not to be a significant predictor of ESRD.[789] A study by Moroni G. et al. found similar prognosis among the focal and mixed categories and these patients had better outcomes compared to crescentic and sclerotic categories of their study subjects.[7] Another study by Tanna et al. found age, eGFR, percentage normal glomeruli and tubular atrophy as important predictors of ESRD in their cohort.[9] European group (Brix et al.) developed and validated a renal risk score based on clinical and pathological factors taking into consideration renal function at time of biopsy along with glomerular crescents and tubulo-interstitial compartment chronicity on a large set (205 patients) of training and validation cohort.[10] Univariate analysis done in this study demonstrated % of normal glomeruli (P = <0.001), percentage of capsular rupture (P 0.003), fibrous crescents (P = 0.013), degree of interstitial fibrosis and tubular atrophy (P = 0.001) and eGFR (P < 0.001) were significant predictors of end stage renal disease. Our study validates the results of this study and we also found eGFR, % normal glomeruli, degree of IF/TA as significant predictors of ESRD. Multivariate Cox regression analysis in our study also confirmed IF/TA (<0.001) and % normal glomeruli (P = 0.018) as important predictors of ESRD as shown in the study by Brix et al. Another study published by Berden et al. in 2012 showed that patients with higher proportion of fibrous crescents, globally sclerosed glomeruli and IF/TA have relatively impaired renal function at one year.[17] Kaplan-Meier survival analysis in our study showed significant difference in survival with respect to low, medium and high risk categories based on the renal risk score and we validate the observations made by Brix et al.[10]
Conclusion
PICGN is a significant cause of mortality and morbidity. Renal histological factors such as % normal glomeruli at time of biopsy, degree of IFTA and renal risk score play an important role in assessing prognosis in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Outcome of ANCA-associated renal vasculitis: A 5-year retrospective study. Am J Kidney Dis. 2003;41:776-84.
- [Google Scholar]
- Primary glomerular diseases. In: Brenner BM, Rector FC, eds. The Kidney (4th ed). Philadelphia, Pa, USA: Saunders; 1991. p. :1182-279.
- [Google Scholar]
- ANCA glomerulonephritis and vasculitis: A Chapel Hill perspective. Semin Nephrol. 2000;20:233-43.
- [Google Scholar]
- Validation of the new classification of pauci-immune glomerulonephritis in a United States cohort and its correlation with renal outcome. BMC Nephrol. 2013;14:210.
- [Google Scholar]
- Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: A study of 121 patients in a single center. Nephrol Dial Transplant. 2012;27:2343-9.
- [Google Scholar]
- Validation of a newly proposed histopathological classification in Japanese patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. BMC Nephrol. 2013;14:125.
- [Google Scholar]
- Predictors of renal survival in ANCA associated vasculitis: validation of a histopatological classification schema and review of the literature. Clin Exp Rheumatol. 2015;33:S.
- [Google Scholar]
- ANCA positive crescentic glomerulonephritis outcome in a Central East European cohort: A retrospective study. BMC Nephrol. 2015;16:90.
- [Google Scholar]
- Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: Evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant. 2015;30:1185-92.
- [Google Scholar]
- Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018;94:1177-88.
- [Google Scholar]
- The prevalence and management of pauci-immune glomerulonephritis and vasculitis in western countries. Kidney Dis (Basel). 2016;1:224-34.
- [Google Scholar]
- Kidney disease. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2015. New York, NY, USA: McGraw-Hill; 2014.
- [Google Scholar]
- Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int. 2003;63:670-7.
- [Google Scholar]
- Histopathological classification of pauci-immune glomerulonephritis and its impact on outcome. Rheumatol Int. 2014;34:1721-7.
- [Google Scholar]
- Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:599-605.
- [Google Scholar]
- Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. JASN. 1996;7:23-32.
- [Google Scholar]
- Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody–associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol. 2012;23:313-21.
- [Google Scholar]







