Translate this page into:
Factors Affecting Mortality in COVID-19 Patients with Pre-Existing Chronic Kidney Disease
Corresponding author: Vishnu Shankar Ojha, Department of General Medicine, All India Institute of Medical Sciences, Patna, Bihar, India. E-mail: vsojha12@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shyama S, Vardhan H, Ojha VS, Biswas R, Ahmad S, Kumar A. Factors Affecting Mortality in COVID-19 Patients with Pre-Existing Chronic Kidney Disease. Indian J Nephrol. 2024;34:643-5. doi: 10.25259/IJN_67_2024
Abstract
The emergence of COVID-19 triggered a global health crisis, sparking concerns within the medical community about its interaction with chronic kidney disease (CKD) and the heightened vulnerability of individuals with compromised renal function to severe viral infection and mortality. This retrospective study encompassed all adult patients with laboratory-confirmed COVID-19 and pre-existing CKD admitted between May 2020 and May 2023. Their demographic data, relevant clinical parameters, and laboratory values were collected. Kaplan-Meier curve analysis and Log Rank test were employed to compare survival times between CKD patients and those developing acute kidney injury (AKI), while Cox regression analyses were conducted to pinpoint factors influencing the hazard of a fatal outcome. The study, involving 150 COVID-19 patient records with pre-existing CKD, revealed that male gender, advanced age, requirement for invasive ventilation, and elevated levels of inflammatory markers such as total leukocyte count, lactate dehydrogenase, C-reactive protein, D-dimer, and IL-6 significantly increased the risk of death. These findings underscore the necessity for tailored care and meticulous management in COVID-19 patients with coexisting CKD, emphasizing the importance of addressing factors such as gender, age, and inflammatory status to mitigate mortality risks effectively.
Keywords
COVID-19
Renal insufficiency
Chronic
Mortality
Acute kidney injury
Risk factors
Introduction
The emergence of COVID-19, caused by the novel coronavirus SARS-CoV-2, has triggered a global health crisis since its identification in late 2019. While primarily recognized for respiratory symptoms, the impact of the virus extends across multiple organ systems. Among these, chronic kidney disease (CKD) has surfaced as a notable risk factor for severe COVID-19 outcomes. The interaction between COVID-19 and CKD has raised concerns, as individuals with compromised renal function may face increased vulnerability to severe viral infection, and mortality necessitates a thorough investigation to guide clinical management and public health strategies.
Materials and Methods
All patients presenting with respiratory symptoms and confirmed COVID-19 through real-time polymerase chain reaction (RT-PCR) and having pre-existing CKD between May 2020 and May 2023 were included in the study. Patients already on maintenance hemodialysis and those having chronic heart failure or liver failure were excluded. The study was approved by the Institutional Review Board at All India Institute of Medical Sciences, Patna, number AIIMS/PAT/IEC/2021/752, dated 29/09/2021.
Results
Of the total 150 patients included, 114 (76%) were males. The mean (SD) age of the participants was 59.9 (13.8) years with a majority, i.e., 83 (55.3%) aged more than 60 years, followed by 63 (42%) belonging to the age group of 30–60 years. One hundred and thirty five (90%) of the patients had hypertension while 110 (73.3%) had diabetes mellitus. Among the study participants, only six (4%) had a severe COVID infection. Four (2.7%) had a previous history of renal transplantation. One hundred and twenty two (81.3%) of the patients required hemodialysis during their hospital stay, and 83 (55.3%) developed acute kidney injury (AKI). Forty six (30.7%) of the participants survived and were subsequently discharged, while 104 (69.3%) died [Table 1].
| Variable | Categories | n (%) |
|---|---|---|
| Gender | Male | 114 (76) |
| Female | 36 (24) | |
| Age | Less than 30 years | 4 (2.7) |
| 30–60 years | 63 (42) | |
| More than 60 years | 83 (55.3) | |
| Comorbidities | Hypertension | 135 (90) |
| Diabetes mellitus | 110 (73.3) | |
| COVID-19 severity | Severe | 6 (4) |
| Nonsevere | 146 (96) | |
| History of renal transplantation | Yes | 4 (2.7) |
| No | 146 (97.3) | |
| Dialysis requirement | Required hemodialysis | 122 (81.3) |
| Did not require hemodialysis | 28 (18.7) | |
| AKI on CKD | Developed AKI | 83 (55.3) |
| Did not develop AKI | 67 (44.7) | |
| Outcome | Discharged | 46 (30.7) |
| Death | 104 (69.3) |
AKI: acute kidney injury, CKD: chronic kidney disease
On Kaplan–Meier Curve analysis, it was seen that the median survival time in patients who developed AKI was 11 (95% CI: 8.639–13.361) days while the median survival time in patients who did not develop AKI was 12 (95% CI: 6.637–17.363) days. Although visual inspection of the curves shows a steeper curve for the patients who developed AKI [Figure 1], the Log rank test revealed that there was no significant difference between the curves [Chi-square=1.098 (at 1 degree of freedom) and P=0.30].
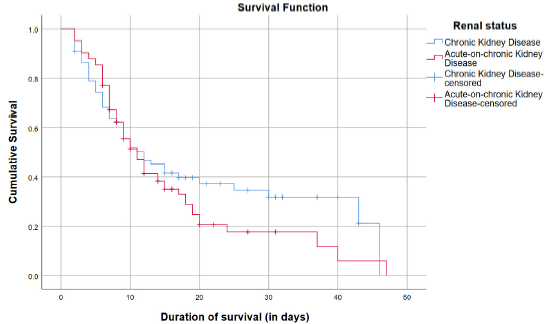
- Kaplan–Meier curves of patients with chronic kidney disease and acute-on-chronic kidney disease.
Univariate cox regression analyses were carried out, and it was seen that male gender, age, requirement for invasive ventilation, increase in total leukocyte count (TLC), lactate dehydrogenase (LDH), C-reactive protein (CRP), D-dimer, and IL-6 levels significantly increased the hazard of death, while the number of total hemodialysis, number of conventional hemodialysis, lymphocytosis, and increased platelet count decreased the hazard of death [Table 2].
| Variable | Categories | Coefficient (B) | Standard Error (SE) | Hazard Ratio (HR) | 95% Confidence Interval of HR | p-value* | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Gender | Female | Ref. | |||||
| Male | 0.636 | 0.257 | 1.888 | 1.141 | 3.123 | 0.013* | |
| Age (in years) | 0.017 | 0.007 | 1.018 | 1.003 | 1.032 | 0.018* | |
| History of renal transplantation | No | Ref. | |||||
| Yes | −0.742 | 0.715 | 0.476 | 0.117 | 1.935 | 0.300 | |
| Dialysis status | No dialysis | Ref. | |||||
| Dialysis performed | −0.076 | 0.251 | 0.927 | 0.567 | 1.516 | 0.762 | |
| Number of total HD | −0.217 | 0.044 | 0.805 | 0.739 | 0.877 | <0.001* | |
| Number of CHD | −0.233 | 0.050 | 0.792 | 0.719 | 0.873 | <0.001* | |
| Number of SLED | −0.133 | 0.238 | 0.875 | 0.513 | 1.493 | 0.625 | |
| Invasive ventilation requirement | No | Ref. | |||||
| Yes | 3.320 | 0.715 | 27.650 | 6.808 | 112.301 | <0.001* | |
| Total leukocyte count | 0.020 | 0.010 | 1.020 | 1.001 | 1.040 | 0.042* | |
| Lymphocytes (%) | −0.061 | 0.022 | 0.940 | 0.900 | 0.982 | 0.006* | |
| Platelets | −0.003 | 0.001 | 0.997 | 0.994 | 0.999 | 0.019* | |
| Albumin | −0.361 | 0.214 | 0.697 | 0.459 | 1.059 | 0.091 | |
| Lactate dehydrogenase | 0.001 | 0.000 | 1.001 | 1.000 | 1.001 | <0.001* | |
| C-reactive protein | 0.001 | 0.001 | 1.001 | 1.000 | 1.002 | 0.031* | |
| D-dimer | 0.019 | 0.009 | 1.019 | 1.002 | 1.037 | 0.028* | |
| Procalcitonin | 0.011 | 0.006 | 1.011 | 1.000 | 1.022 | 0.060 | |
| Serum ferritin | 0.000 | 0.000 | 1.000 | 1.000 | 1.000 | 0.065 | |
| Serum creatinine | 0.000 | 0.001 | 1.000 | 0.999 | 1.002 | 0.643 | |
| Interleukin 6 (IL-6) | 0.000 | 0.000 | 1.000 | 1.000 | 1.001 | 0.012* | |
Discussion
In our study, there was a significant rise in D-dimer levels in nonsurvivors of COVID-19, consistent with findings reported by Tang N. et al.1 This elevation may be attributed to disseminated intravascular coagulation (DIC) caused by sepsis. In sepsis, endothelial cells and monocytes release cytokines, causing free thrombin circulation and platelet activation, indicating activation of the common coagulation pathway.1 The higher hazards ratio for invasive ventilation signified the critical status of patients necessitating invasive ventilation and increased mortality.
We observed that 55.3% of CKD patients with COVID-19 developed AKI, which may be attributed to the direct infection mechanism of the virus. SARS-CoV-2 enters cells through angiotensin converting enzyme (ACE 2), and as it circulates systemically, the kidneys—abundant in ACE 2—emerge as a potential site for viral entry, potentially causing AKI.2
In our study, older male participants faced a heightened mortality risk, potentially linked to higher ACE 2 levels in elderly males.3 Notably, factors like smoking, not considered in our study, may also contribute to decreased male immunity, further escalating their risk of mortality. Age demonstrated a 1.018-fold hazard increase per year in CKD patients, which may be because of compromised viral control and prolonged proinflammatory responses.4
Interestingly, it was observed that lymphocytosis was a protective factor with a hazard ratio of 0.9, while an increase in the TLC was found to increase the hazard of death. This may be due to the fact that lymphopenia has been frequently associated with severe COVID-19 infection.5 Several potential mechanisms have been proposed to elucidate this connection. These mechanisms encompass direct lymphocyte inhibition, destruction of lymph nodes, the release of cytokines, suppression of lymphocytes due to lactic acidosis, and the attachment of the virus to the ACE 2 receptors on lymphocytes.6
Factors such as male gender, advanced age, elevated inflammatory markers, and the necessity of invasive ventilation were associated with an increased risk of mortality in COVID-19 patients with coexisting CKD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury in COVID-19. Int J Mol Sci. 2021;22:8081.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: A retrospective study. Chin Med J (Engl). 2020;133:1261-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Relative lymphocytosis in COVID-19 - a ray of hope. Adv Respir Med. 2020;88:287-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








