Translate this page into:
Fluoroscopy and CT Guided Translumbar Tunneled Dialysis Catheter for Hemodialysis Access Failure in a Case of Autosomal Dominant Polycystic Kidney Disease
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Vascular access in hemodialysis is essential to end-stage renal disease (ESRD) patients’ survival. Unfortunately, even after years of recent advances, a significant number of patients may develop multi-access failure for many reasons. In this situation, arterial-venous fistula (AVF) or catheters placement in traditional vascular sites (jugular, femoral, or subclavian) are not feasible. In this scenario, translumbar tunneled dialysis catheters (TLDCs) may be a salvage option. The use of central venous catheters (CVC) is associated with an increased incidence of venous stenosis that can progressively limit future vascular access routes. The common femoral vein can be used for temporary access in patients in whom traditional approaches for permanent central venous access may not be feasible because of either chronically occluded or not accessible vasculature; however, this location is not preferred for long-term venous access because of the high rate of catheter related blood stream infections (CRBSI). In these patients, a direct translumbar approach to the inferior vena cava is a lifesaving alternative. This approach has been described by several authors as a bail-out option. Fluoroscopy-guided access via a translumbar approach into the inferior vena cava bares the risk of hollow-organ perforation or severe bleeding from the inferior vena cava or even the aorta. To minimize the risk of complications caused by a translumbar central venous access, we hereby present a hybrid approach with CT-guided translumbar access of the inferior vena cava followed by a conventional implantation of the permanent central venous catheter. CT scan-guided access of IVC that further helps in our case as patient has large bulky kidneys secondary to autosomal dominant polycystic kidney disease.
Keywords
Hemodialysis
translumbar
tunneled dialysis catheter
Case Report
A 48-year-old female housewife by occupation and a known case of type II diabetes mellitus controlled with medication and currently with no other diabetes-related complications was referred to the Department of Vascular Interventional Radiology for hemodialysis multi-access failure by the Department of Nephrology. In 2018, she was initiated with hemodialysis. Maintenance hemodialysis was continued via multiple access sites initially from jugular and femoral temporary and permanent tunneled catheters. There was a history of non-maturation and failure of AV fistulae in both upper limbs. At present, on ultrasound doppler screening, there is chronic thrombosis of bilateral internal jugular veins and femoral veins. CT venography neck and mediastinum revealed bilateral brachiocephalic venous and/or superior vena caval occlusion and the absence of large accessible thoracic collateral veins for central venous catheter placement. With this clinical scenario, translumbar and transhepatic dialysis catheter insertion were the available options. The Translumbar route was preferred over the transhepatic route in view of innumerable cysts within the liver parenchyma secondary to polycystic liver disease [Figure 2]. Translumbar dialysis catheter (TLDC) in our case was placed in an angiography suite by using fluoroscopic and Dyna CT scan guidance [Figure 1]. Coagulation parameters (partial thromboplastin time, prothrombin time, and platelet count) were checked and corrected before procedure as necessary. TLDC insertion was performed under local anesthesia with mild sedation. Prophylactic antibiotics were administered before catheter placement. Breathing, pulse, blood pressure, and oxygen saturation were monitored during all the procedures. Patient and relatives were counselled about the procedure, and informed consent was obtained. BARD GLIDE CATH 35-cm catheter with 14.5 French diameter was used. The patient was placed in prone decubitus position, and the skin prepared and draped from below the iliac crest to the lower ribs and from the spine to the mid abdomen. Chiba needle 21-G, a 15-cm-long needle, was advanced under fluoroscopic and DynaCT guidance through subcutaneous tissues and back muscles (erector spinae and psoas) toward the inferior vena cava (IVC) [Figure 2]. Once the needle entered the IVC, a guidewire was passed through it [Figure 3 and 4], and COOK percutaneous access set was used to secure the access. The translumbar catheter was ultimately advanced until the tip is in the right atrium. Dacron cuff was tunneled under the skin and sutured [Figure 4 and Figure 5]. The translumbar exit site was placed above the beltline and as far anterior as possible for patient comfort. DynaCT was performed for confirming the course of translumbar catheter [Figure 6]. After placement of the TLDC, the patient complained of leg and back pain for 2 days and was managed with mild analgesics. Patient underwent dialysis on the same day of catheter insertion. A blood flow rate of 320 ml/min was achieved.

- Schematic diagram showing trans lumbar dialysis catheter
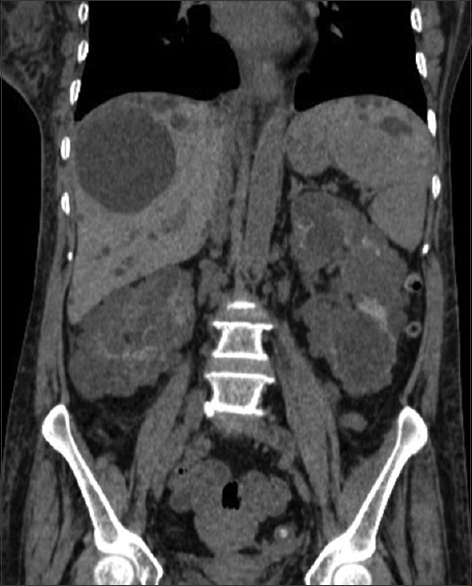
- Coronal CT scan plain abdomen showing polycystic appearance involving both kidneys and liver
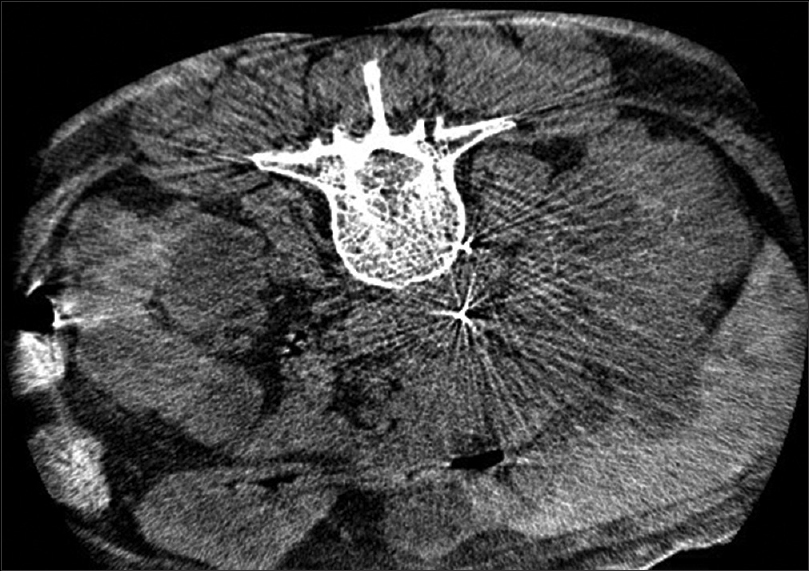
- Dyna CT scan plain abdomen showing the tip of Chiba needle within the IVC
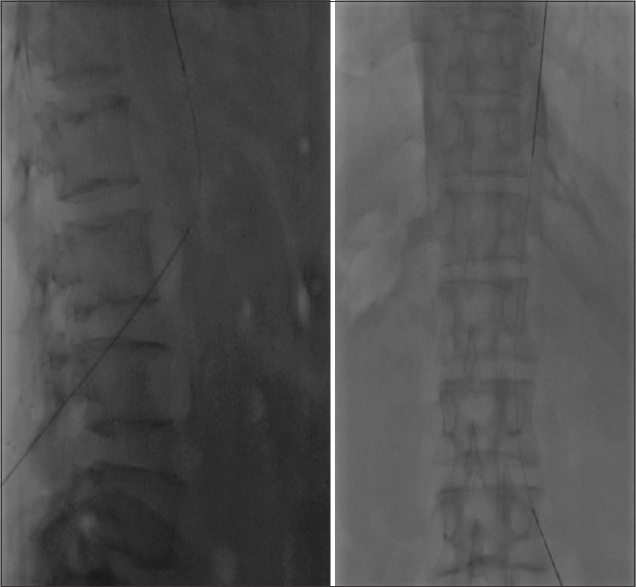
- AP and lateral fluoroscopy image showing the tip of Chiba needle at the level of L3 vertebral body with wire within the IVC

- AP radiograph showing trans lumbar tunneled dialysis catheter with tip of the catheter at cavo-atrial junction

- Post-procedure CT scan showing the course of translumbar dialysis catheter entry into psoas muscle and later into IVC
Discussion
Non-tunneled central vein catheters (CVCs) are temporary means of dialysis. The AVF requires at least 2 months to mature for optimal dialysis. The TDC serves as a bridge to AVF dialysis. The National Kidney Foundation Dialysis Outcomes Quality Initiative (NKF-KDOQI) recommends that less than 10% of chronic maintenance hemodialysis patients are maintained on catheters as their permanent dialysis access. In certain clinical situations, such as the lack of suitable vessels available for AVF or the inability to use these fistulae due to complications such as thrombosis or bleeding or any other cause, the use of TDCs/permanent catheters has been crucial. CV catheters can be non-tunneled or tunneled subcutaneously prior to entering the vein.[1,2] Patients in whom traditional approaches for permanent central venous access may not be feasible because the vasculature is chronically occluded or not accessible. In these cases, the common femoral vein can be used for temporary access; however, this location is not preferred for long-term venous access because of the high rate of infections. In these patients, a direct translumbar approach to the inferior vena cava is an alternative. This approach has been described by several authors as a bail-out option.[3] The translumbar approach is generally done under fluoroscopy guidance; however, in certain scenarios, there is a need for fluoroscopy and cross-section guidance.
It is important not to enter the IVC perpendicular to its long axis as this would make advancing the guidewire toward the right atrium difficult, as well as predisposing the peel-away sheath and catheter to kinking. As soon as the needle entered the abdominal cavity, which was felt by the loss of resistance as the needle goes through the abdominal wall, DynaCT was used in our case to confirm its location and trajectory. If everything is appropriately aligned, the needle can be advanced further toward the IVC with another CT performed once the needle is thought to be close to the outer wall of the IVC. CT navigation enables better puncture trajectory planning and on-line needle control with sparing of nearby structures, especially the aorta, kidneys, ureter, and intestines, which in patients with atrophic kidneys, extend farther dorsally into the retroperitoneum region. One must keep in mind that the puncture canal is dilated gradually up to size 16 F and that a potential retroperitoneal structure injury can have serious consequences. In our case, the patient had large bulky kidneys with polycystic appearance; thus, for optimal puncture trajectory, the DynaCT part of the DSA suite was used for navigation. DynaCT can be performed directly in the angiography suite without loss of time and additional risk to the patient. The use of DynaCT also prevents the transfer of patients from the CT scan room to the catheterization laboratory. Venous variants are more common than arterial anatomic variations and can be diagnosed more easily with the use of CT. Presently, CT navigation is used in more centers than ever before. Originally, the TLDC were inserted under fluoroscopic control by using anatomic landmarks, centering the needle at the L2–L3 vertebral bodies level. Occasionally, the catheter or wire is inserted into the IVC beforehand to serve as the target point for the puncture. There are no reports in the literature comparing these two approaches, but in fluoroscopy-guided IVC punctures, the complication rate is approximately 10%. Recently, results on TLC placement relying on cone-beam CT navigation were published. This technique is performed in the angiography suite and, by integrating puncture and catheter insertion in a single location, it eliminates the need for patient transfer from the CT intervention room to the angiography suite.[4]
Some studies discourage TLDC use because of low patency. Liu et al.[5] observed catheter patency at 3, 6, and 12 months of 43%, 25%, and 7%, respectively. Poor patency rate was attributable to the high rate of late thrombosis. Through the use of fluoroscopy and CT guidance, the placement of the tip of the catheter is confirmed, making sure that it does not abut the vessel wall, and the top of the catheter is evaluated to ensure a smooth curve without any kinks. The tug test refers to the rapid flow of blood when a 10-mL syringe is attached to both venous and arterial ports and vigorously flushed. Median blood flow rate should be 300–350 mL/min. According to the authors, this is considered an adequate flow for a translumbar device. Lund et al. defined translumbar catheter failure as a blood flow rate of less than 200 mL/min. (14) In this situation, some interventions are necessary to assess eventual thrombus formation and/or improper catheter positioning. Such measures include clot dissolving medication (urokinase) and venography with catheter repositioning. Catheter removal or replacement must be done only when these measures prove unsuccessful.[1,4,5]
We used the definitions proposed by the Society of Interventional Radiology (SIR). According to those definitions, the initial (primary) device service interval is defined as the number of catheter days from TLDC insertion until device failure or removal at the completion of therapy, patient death, or conclusion of the study with the catheter still functioning. Revised (secondary) device service interval is defined as the service interval that begins after device replacement or salvage, without abandonment of the access site. Device failure is defined as any limitation in catheter function despite a technically successful placement. The target blood flow for the central venous catheter is ≥300 mL/min.[5-7]
Although generally safe and associated with low rates of complications, TLDC still has a higher rate of long-term complications, replacements, and removals than traditional tunneled dialysis catheters. Among the most reported complications are catheter-related infection and thrombosis. Gregory et al. reported that the main indications for exchange or TLDC removal (n = 78) were catheter-related infection (n = 39; 50.0%), catheter malposition (n = 15; 19.2%), catheter malfunction secondary to occlusion (n = 10; 12.8%), mature permanent vascular access (n = 7; 9.0%), conversion to peritoneal dialysis (n = 3; 3.9%), functioning transplant (n = 2; 2.6%), malfunction and infection (n = 1; 1.3%), and unknown (n = 1; 1.3%). The reported cumulative 12-month catheter patency rates ranged from 7% to 73.2%. (4) Some interventions may be necessary to evaluate and correct the eventual formation of thrombus and/or inadequate catheter position. Such measures include the use of a thrombolytic agent and venography-guided catheter repositioning, respectively. With the increase of hemodialysis patients’ life expectancy and the low availability of kidney donors, maintaining vascular access is critical and often challenging. When performed by a specialized team, fluoroscopy and CT-guided placement of TLDC is a safe procedure. If successful, TLDC can be a life-saving option in patients with access failure as a bridge to a long-term solution such as kidney transplantation.[8-11]
To perform catheter exchanges, a single guidewire was passed through the catheter into the right atrium. After caval access was preserved, the catheter was removed, and a new catheter was introduced over the wire using the weaving technique, in which a single guidewire was passed through the distal tip and then passed into the more proximal tip before being advanced over the wire. Catheter removal was accomplished using the traction technique, in which gentle sustained traction is applied to the catheter to free the incorporated cuff from the subcutaneous tissues. Compression was given at the puncture site. Removal of the catheter, when necessary, is performed after sterilization of the skin with surgical scrubbing solution, removal of the skin suture (if any), and freeing of the Dacron cuff with blunt dissection. Hemostasis was achieved with manual compression at the insertion site followed by supine positioning of the patient. Patients have no clinical or laboratory evidence of bleeding after catheter removal with normal bleeding parameters.[4,12,13]
Conclusion
In summary, TLDC placement is feasible and relatively safe. It is a salvage life-saving procedure to allow the patients to be on maintenance hemodialysis and should be used as a bridge to kidney transplantation. The presented CT-guided translumbar approach followed by a fluoroscopy-guided placement permanent catheter represents a safe bail-out option for patients suffering from chronically occluded veins routinely used as an access site for dialysis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Central venous access: A primer for the diagnostic radiologist. Am J Roentgenol. 2002;179:309-18.
- [Google Scholar]
- Recent trends in central venous catheter placement: A-comparison of interventional radiology with other specialties. J-Vasc Interv Radiol. 2001;12:1211-4.
- [Google Scholar]
- CT-guided translumbar placement of permanent catheters in the inferior vena cava: Description of the technique with technical success and complications data. Cardiovasc Intervent Radiol. 2018;41:1356-62.
- [Google Scholar]
- Translumbar hemodialysis catheters in patients with limited central venous access: Does patient size matter? J Vasc Interv Radiol. 2013;24:997-1002.
- [Google Scholar]
- Patency and complications of translumbar dialysis catheters. Semin Dial. 2015;28:E41-7.
- [Google Scholar]
- Translumbar hemodialysis long-term catheters: An alternative for vascular access failure. Braz J Nephrol 2018 doi:10.1590/2175-8239-jbn-2018-0080
- [Google Scholar]
- Translumbar central venous catheters for long-term haemodialysis. Nephrol Dial Transplant. 2009;25:1588-95.
- [Google Scholar]
- Hemodialysis reliable outflow-(HeRO) device in end-stage dialysis access:A-decision analysis model. J-Surg Res. 2012;177:165-71.
- [Google Scholar]
- Translumbar hemodialysis catheters in patients with limited central venous access: Does patient size matter? J Vasc Interv Radiol. 2013;24:997-1002.
- [Google Scholar]
- Translumbar hemodialysis long-term catheters: An alternative for vascular access failure. Braz J Nephrol. 2018;41:89-94.
- [Google Scholar]
- Vascular access for placement of tunneled dialysis catheters for hemodialysis: A-systematic approach and clinical practice algorithm. J-Clin Imaging Sci. 2015;5:31.
- [Google Scholar]
- Placement of long-term hemodialysis catheter (permcath) in patients with end-stage renal disease through external jugular vein. Adv Biomed Res. 2014;3:252.
- [Google Scholar]







