Translate this page into:
Gastrointestinal Involvement in Granulomatosis with Polyangiitis: Case Report and Review
Address for correspondence: Dr. Raja Ramachandran, Department of Nephrology, Post Graduate Institute of Medical Education and Research, Chandigarh - 160 012, India. E-mail: drraja_1980@yahoo.co.in
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Granulomatosis with polyangiitis (GPA) commonly affects upper/lower respiratory tract and kidneys. It causes necrotizing vasculitis of small and medium-sized blood vessels. Gastrointestinal (GI) involvement is an uncommon manifestation of GPA, and presentation with predominant GI manifestation is noteworthy. We report a case of 50-year-old male with melena due to GI vasculitis along with other systemic involvement. The patient was treated with pulse methylprednisolone, cyclophosphamide, and plasmapheresis. To manage the refractory GI bleed, the patient underwent surgical resection, and the histology of the surgical specimen confirmed necrotizing vasculitis.
Keywords
ANCA vasculitis
gastrointestinal manifestation
granulomatosis with polyangiitis
Introduction
Granulomatosis with polyangiitis (GPA) is a small and medium vessel vasculitis with a predilection for respiratory tract and kidneys. It causes fibrinoid necrosis of small and medium-sized vessels leading to organ dysfunction. Gastrointestinal (GI) involvement is seen in only 5–10% of the cases and is a poor prognostic factor.[1] Both small and large bowel can be affected leading to life-threatening complications. We report a case of GPA with GI involvement in a middle-aged man who presented with a severe lower GI bleed and died despite aggressive management.
Case Report
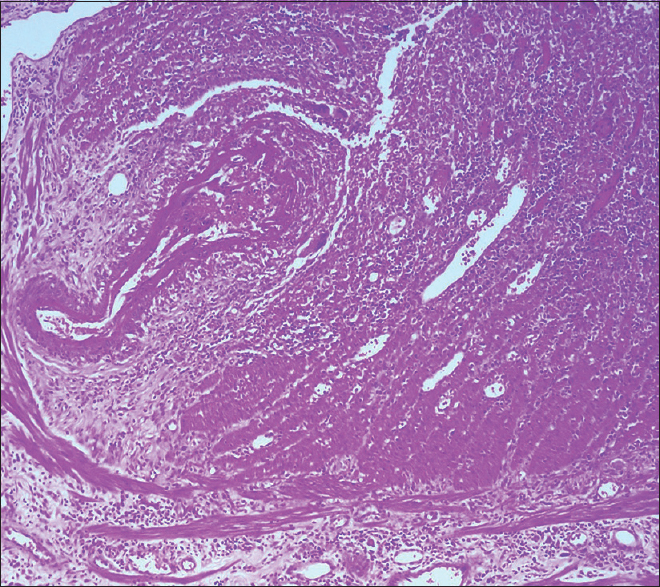
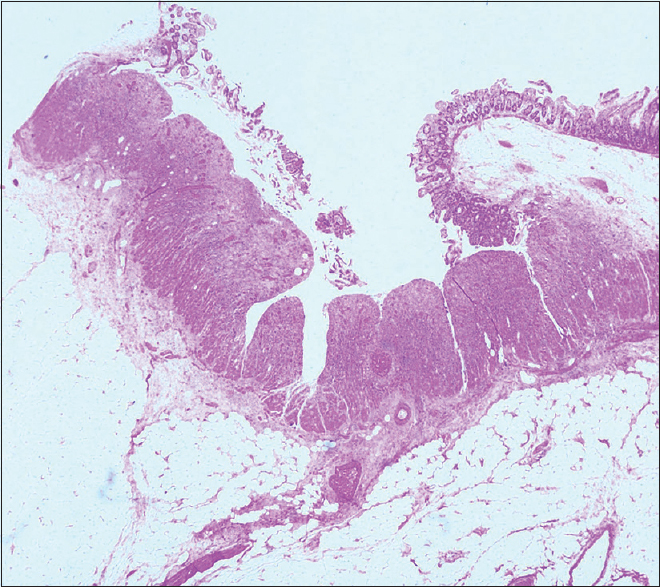
A 50-year-old man, with a history of old pulmonary tuberculosis and a smoking index of 40 presented to otorhinolaryngology services with complaints of vertigo and decreased hearing. He was managed as a case of bilateral otitis media with a temporary improvement of symptoms, but subsequently developed left facial palsy a month later and underwent left cortical mastoidectomy. He developed pain and chemosis of the right eye after 2 months, which was successfully managed with parenteral antibiotics. Contrast-enhanced magnetic resonance imaging of the brain was normal. After 15 days, the patient presented with complaints of oral ulcers, fever, and lower GI bleed. There were 5–6 episodes of black stools mixed with fresh blood associated with crampy, diffuse, intermittent abdominal pain, which was not relieved by passing stool. In the past, he was diagnosed to have pulmonary tuberculosis and had completed anti-tuberculous treatment. On examination, he was conscious and oriented. His blood pressure was 124/60 mm Hg right arm supine with a pulse rate of 108/min. He was pale, had ulcers in the oral cavity [Figure 1], and small discrete papular lesions on the dorsum of the right hand [Figure 2]. Skin biopsy done from lesions was suggestive of leukocytoclastic vasculitis with negative immunofluorescence. Systemic examination was noncontributory. On evaluation, he had severe anemia (Hb, 3.8g/dl), leukocytosis (31000/cu mm) and thrombocytosis (platelet, 7.9 × 106/cu mm). His serum creatinine and serum albumin level were 6.8 mg/dl and 1.9 gm/dl, respectively. Routine urine examination revealed 1+ albuminuria, 35–40 erythrocytes/high-power field with erythrocyte casts. Liver function test and coagulation profile were normal. The antinuclear factor was 2+ and cytoplasmic anti-neutrophilic cytoplasmic antibody was 3+ positive. Ultrasonography revealed mild ascites with septations. Thickened bowel loop was seen in the left iliac region, with a maximum thickness of 7 mm with loss of surrounding gut signature. Both kidneys were normal in size. Given the suspicion of GI bleed, contrast-enhanced computerized tomography of the chest and abdomen was done, which revealed nodules in the bilateral lung fields, dilated proximal bowel loops, and a collapsed large intestine, suggestive of a terminal ileal stricture. With a high clinical suspicion of systemic vasculitis, the patient underwent colonoscopy, which showed multiple ulcers of the colon with mucosa smeared with blood. The patient was given injection methylprednisolone for 3 days (1g each) followed by intravenous cyclophosphamide (1g) pulse. The patient continued to have a drop in hemoglobin despite frequent blood transfusions. Digital subtraction angiography was done to localize the site of bleed, which revealed extravasation into jejunum from a branch of the superior mesenteric artery. Given active bleed, the patient underwent emergency surgery. The intraoperative finding revealed multiple telangiectasia starting from distal jejunum to distal ileum with an ulcer 10 cm proximal to the ileocecal junction was seen. The resected specimen showed multiple large ulcers with a perforation, which on microscopy showed fibrinoid necrosis [Figures 3 and 4].

- Oral ulcer in the soft palate with surrounding erythema
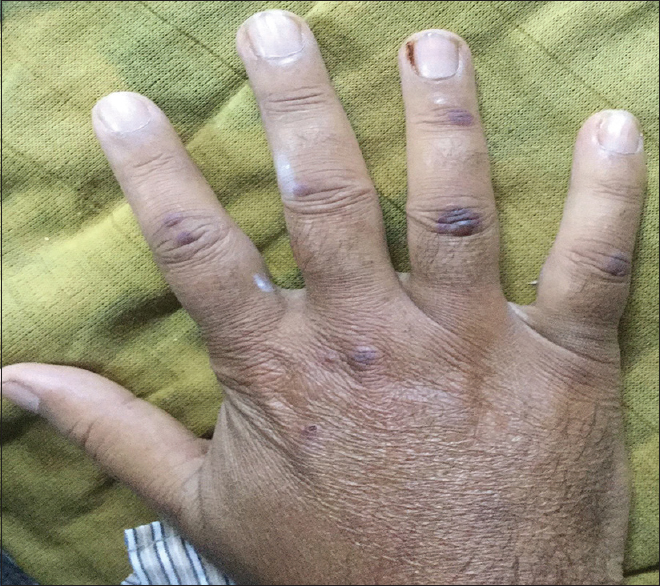
- Papular eruption in the right ringfinger
- Small intestine showing large ulcers with undermining edges dipping into the submucosa
- Blood vessel showing evidence of fibrinoid necrosis and fibrin thrombi
The patient continued to have downhill course post-surgery and required frequent blood transfusions and continued to have a rectal bleed. Rapidly progressive renal failure (serum creatinine increased from 1.9 to 7.6 in a week) prompted us to commence plasma exchange and hemodialysis. However, the patient developed hypotension and sustained a refractory cardiac arrest.
Discussion
In the current report, we highlight an uncommon presentation of GPA presenting with GI bleed and intestinal perforation. Despite aggressive management with steroids, cyclophosphamide, plasmapheresis, and surgery, the prognosis was dismal.
GPA is a medium and small vessel vasculitis, which affects the respiratory tract and the kidney. Necrotizing granulomatous inflammation characterizes the clinical condition. Five to ten percent of the cases have GI involvement.[1] The clinical spectrum is highly variable, and vasculitis may be systemic or localized. GI tract involvement is often a part of a systemic inflammatory process and is a well-recognized manifestation of small- and medium-sized vessel vasculitis. Patients with GI involvement usually present with abdominal pain, nausea/vomiting, diarrhea, or GI bleed. When present, GI complications adversely affect prognosis and are an indicator of disease severity.[2]
Our index case was a middle-aged man. Although GPA occurs with equal frequency in males and females, GI manifestations are seen more often in males. He had bilateral otitis media, nodules in lungs and right eye chemosis, and later presented with renal failure and lower GI bleed. The clinical presentation is similar to prior observation.[2] In the past, diverse GI lesions including ulcers, submucosal edema, hemorrhage, paralytic ileus, mesenteric ischemia, bowel obstruction, and intestinal perforation have been reported.[3] In the present case, small intestine mainly jejunum and ileum were involved. Moreover, the patient had positive C-ANCA, which was consistent with GPA. The biopsy of the resected intestine was suggestive of necrotizing inflammation similar to prior reports.[4] The patient had tested positive for antinuclear factor, which we attribute to false positivity, as the dsDNA antibody was negative and complement C3 and C4 were normal. Skaife et al. in their study postulated that vasculitis could cause ischemia and lead to perforation, which we also hypothesize to have occurred in our case as well.[4]
American College of Rheumatology (1990) defines GPA by the presence of at least two of the four criteria: (1) nasal or oral inflammation, (2) abnormal chest radiograph with either the presence of nodules, fixed infiltrates, or cavities, (3) urine sediment with hematuria or red cell cast, and (4) granulomatous inflammation on biopsy within an artery or in the perivascular or extravascular area of an artery or arteriole.[5] The index case presented at least two of the four criteria. Given an organ-threatening disease, the index case underwent treatment with pulse methylprednisolone, cyclophosphamide, and plasmapheresis. Moreover, he underwent ileal resection. Unfortunately, the disease proved fatal despite aggressive management. Poor prognosis with intestinal perforation is well known. Intestinal perforation in GPA is a rarer manifestation of the disease, and till date, at least 11 cases have been reported in the literature. Patient's demographic, clinical profile, and outcomes with intestinal perforation are mentioned in Table 1. The mean age of patients having ANCA-related perforation was 48 years. The most common sites of perforation were jejunum, ileum, stomach, and esophagus.[3] The small bowel was involved in all cases with ileal involvement in 9 (82%) cases. Ulceration and perforation are the most common pathologies. Microscopically, most examples show nonspecific inflammation and ulceration. These nonspecific inflammations found in biopsies are a result of the biopsy taken too superficially. Only three cases had characteristic granulomas diagnostic of GPA. C-ANCA was positive in all patients with documented status, which is consistent with the present report.
| Cases (Year) | Age/sex | Gastrointestinal site | Pathology | Biopsy | Renal involvement | ANCA | Surgery | Gastrointestinal involvement at onset | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Shamim et al., 2010[6] | 35/M | Jejunum | Perforation | Inflammation ulceration | Y | C-ANCA | Y | N | Survival |
| Alexander et al., 2015[7] | 56/F | Esophagus, ileum | PerforationInflammationUlceration | Inflammation nuclear debris | Y | C-ANCA | Y | Y | Death |
| Shaikh et al., 2006[8] | 44/F | Distal jejunum,ileum, colon | Perforation | Vasculitis, necrosis | N | C-ANCA | Y | N | Survival |
| Skaife et al., 2000[4] | 69/M | Distal jejunum | Perforation | Vasculitis | Y | C-ANCA | Y | Y | Death |
| Bulus et al., 2013[9] | 47/F | Jejunum, ileum | Perforation Necrosis | Necrotizing granulomatousVasculitis | Y | C-ANCA | Y | Y | Death |
| Akça et al., 2005[10] | 56/M | Distal ileum | Necrosis,Perforation | Ulcerations, inflammation,fistula, fibrosis | N | C-ANCA | Y | N | Survival |
| Strivens et al., 2005[11] | 54/M | Distal ileum,Colon | Perforation | Vasculitis | Y | C-ANCA | Y | N | Survival |
| Deniz et al., 2007[12] | 44/M | Ileum | Perforation | Ulcerations, necrotizinggranulomas, vasculitis | N | C-ANCA | Y | N | Survival |
| Yildirim et al., 2010[13] | 32/M | Ileum | Perforation | Inflammation, necrotizinggranulomatous vasculitis | N | C-ANCA | Y | N | Death |
| Akbulut et al., 2012[14] | 47/F | Ileum | Perforationfistula | NA | N | NA | Y | N | Death |
| Dinić et al., 2013[15] | 52/F | Small bowel | Perforation | NA | Y | C-ANCA | Y | N | Death |
| Index Case | 50/M | Jejunum and Ileum | Perforation ulceration | Ulceration Fibrinoid necrosis vasculitis | Y | C-ANCA | Y | Y | Death |
NA: Not available, M: Male, F: Female, Y: Yes, N: No, C-ANCA: Cytoplasmic anti neutrophilic antibody
Three patients out of 11 had GI manifestation as the presenting symptom. Masiak A et al. also concluded in their study that GI manifestation can be the first symptom of GPA and predicts involvement of other organ systems.[3] Though GI involvement is usually a part of the systemic disease, in our review, only 6 (54.5%) patients had renal involvement. Almost all patients required surgical intervention, with a mortality rate of 54.5%.
To conclude, we present a case of GI ulceration and perforation as a presenting manifestation of GPA, which carried a poor prognosis despite optimal management.[15]
Learning points
-
Although rare, massive GI ulceration and perforation can be a presenting manifestation in patients with GPA
-
GI bleed in GPA is a result of the underlying disease and/or aggravated by concurrent immunosuppressive therapy. GI bleed can be potentially catastrophic and warrants a high index of suspicion, close monitoring, and aggressive management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: Analysis of 62 patients with polyarteritisnodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore). 2005;84:115-28.
- [Google Scholar]
- Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med. 1992;116:488-98.
- [Google Scholar]
- Gastrointestinal tract involvement in granulomatosis with polyangiitis. Gastroenterol Rev. 2016;11:270-5.
- [Google Scholar]
- Intestinal perforation as a presentation of Wegener's granulomatosis. Hosp Med. 2000;61:286-7.
- [Google Scholar]
- The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101-7.
- [Google Scholar]
- Intestinal perforation as an early complication in Wegner's granulomatosis. World J Gastrointest Surg. 2010;2:169-71.
- [Google Scholar]
- Esophageal vasculitis in granulomatosis with polyangiitis. TropGastroenterol. 2015;36:132-4.
- [Google Scholar]
- Extensive intestinal ischaemic necrosis in Wegener's granulomatosis. Gut. 2006;55:1368-9.
- [Google Scholar]
- Intestinal perforation as the initial presentation of Wegener's granulomatosis. RheumatolInt. 2013;33:2957-8.
- [Google Scholar]
- Intestinal perforation in Wegener's granulomatosis: A case report. UlusTravmaAcilCerrahiDerg. 2005;11:348-51.
- [Google Scholar]
- Intestinal perforation and jejunalhaemorrhage due to Wegener's granulomatosis. ClinExpRheumatol. 2005;23:124.
- [Google Scholar]
- Intestinal involvement in Wegener's granulomatosis. J Gastointestin Liver Dis. 2007;16:329-31.
- [Google Scholar]
- Multiple intestinal perforation in a patient with Wegener's granulomatosis: A case report and review of the literature. GastroenterolClinBiol. 2010;34:712-5.
- [Google Scholar]
- Multiple Ileal perforations in a patient with Wegeners granulomatosis: A case report and literature review. J GastrointestSurg. 2012;16:857-62.
- [Google Scholar]
- Fulminant Wegener's granulomatosis: A case report. VojnosanitPregl. 2013;70:887-90.
- [Google Scholar]







