Translate this page into:
Glomerular Diameter Measurements on Light Microscopy: A New Parameter Available to Pathologists and its Utility in IgA Nephropathy
Corresponding author: Swarnalata Gowrishankar, Department of Histopathology, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, India. E-mail: swarnalatag@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Manocha A, Fathima N, Gowrishankar S. Glomerular Diameter Measurements on Light Microscopy: A New Parameter Available to Pathologists and its Utility in IgA Nephropathy. Indian J Nephrol. 2024;34:344-9. doi: 10.25259/ijn_361_23
Abstract
Background:
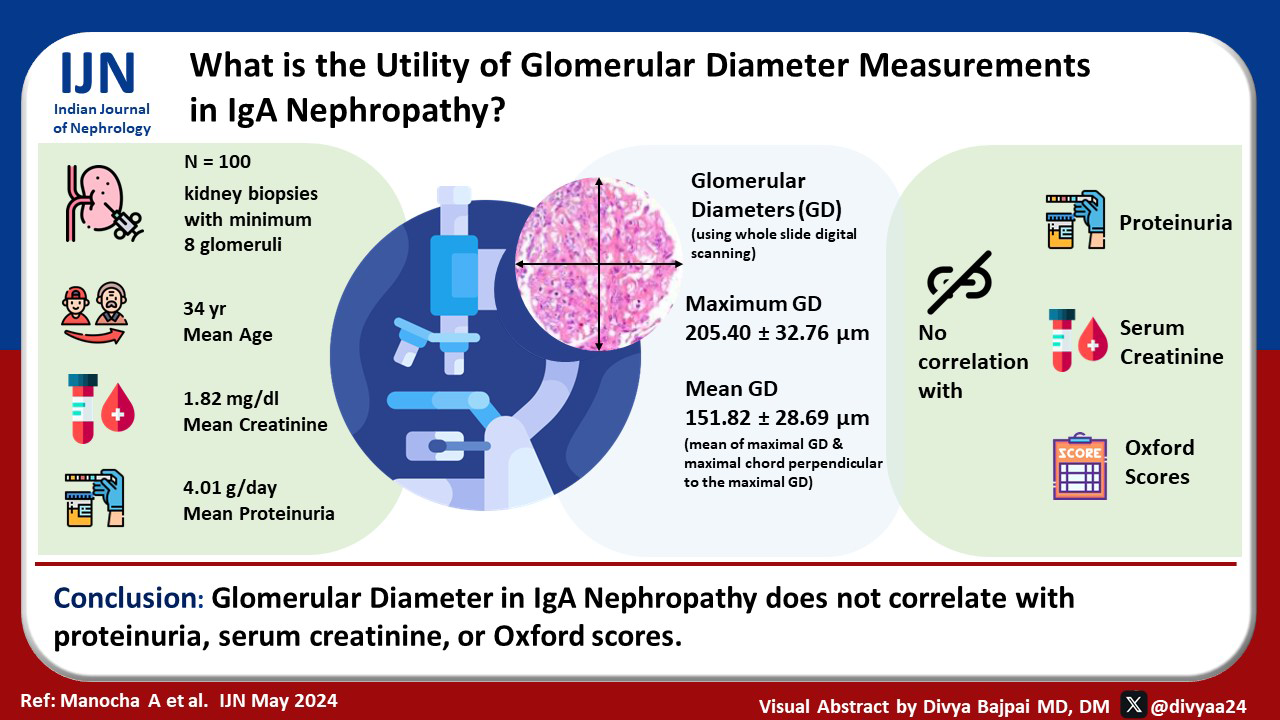
With the availability of whole slide digital scanners, fairly accurate glomerular diameter (GD) measurements are now possible on light microscopy. The value of these measurements in prognosis and diagnosis of immunoglobulin A nephropathy (IgAN) have not been studied widely. IgAN is a major cause of end-stage renal disease (ESRD) worldwide, and its progression is currently assessed using Oxford scores, serum creatinine, and 24-h urinary protein. We aimed to correlate the mean and maximum GDs with serum creatinine, 24-h urinary protein, and Oxford scores in patients with IgAN.
Materials and Methods:
One hundred biopsies of IgAN with a minimum of eight viable glomeruli were collected along with data of their 24-h proteinuria, serum creatinine, and Oxford scores. The slides were scanned using the Philips IntelliSite Pathology Solution-Ultra Fast Scanner. Mean GD of each glomerulus was calculated as the mean of two measurements, that is, the maximal diameter of the glomerulus and the maximal chord perpendicular to the maximal diameter. Maximum GD was also recorded for each case. The Spearman rho/Pearson R correlation coefficient was used to make this correlation. P-values <0.05 were considered statistically significant.
Results:
The mean age of the patients was 34.67 ± 12.03 years, and they showed a male preponderance. The overall mean GD was 151.82 ± 28.69 µm, and maximum GD was 205.40 ± 32.76 µm. No statistically significant correlation was observed between the mean or maximum GD and the 24-h proteinuria, serum creatinine levels, and Oxford scores.
Conclusion:
GD in IgAN does not correlate with proteinuria, serum creatinine, or Oxford scores.
Keywords
Digital pathology
Glomerular diameter
IgA nephropathy
Proteinuria
Whole slide imaging
Introduction
Immunoglobulin A nephropathy (IgAN) is a major cause of the end-stage renal disease (ESRD) worldwide.1 Several studies have investigated the histological features of IgAN in a renal biopsy for correlation with clinical outcome.2,3 The progression of IgAN is currently assessed using the Oxford MEST-C score (mesangial and endocapillary hypercellularity, segmental sclerosis, interstitial fibrosis/tubular atrophy, and crescents).4 However, this score has been found to have a low prognostic ability cumulatively, as well as for each score individually.4 Many studies have consistently found that glomerulosclerosis and interstitial fibrosis with tubular atrophy are indicators of poor prognosis in IgAN. However, neither are these findings specific for IgAN nor can indicators of advanced chronic renal disease be used to guide treatment.5 Other markers used to assess severity are serum creatinine and 24-h urinary protein, which are nonspecific.4
The process of digitization of glass slides along with the development of specialized software for tissue image analysis has resulted in the generation of precise and reproducible tissue-derived readouts.6 Thus, whole slide imaging can provide fairly accurate glomerular diameter (GD) measurements. There are very few studies in literature assessing the predictive value of glomerular size in IgAN. This is based on the hypothesis that kidneys with reduced nephron number, presumably having less functional reserve, cause a glomerular enlargement, resulting in increased susceptibility to subsequent renal injury and functional decline.7 In this study, we aimed to correlate the mean and maximum GD with serum creatinine levels, 24-h urinary protein, and Oxford MEST-C scores in patients with IgAN.
Materials and Methods
We reviewed 100 biopsies diagnosed as IgAN. These biopsies were received between April 1, 2022 and January 1, 2023 in the Department of Histopathology at Apollo Hospitals. The study was approved by the Institutional Review Board at Apollo Hospitals, Jubilee Hills, Hyderabad, number AHJ-ACD-019/09-22, dated 29th July 2022. The inclusion criteria were the presence of ≥8 viable glomeruli in the biopsy as well as the availability of serum creatinine and 24-h proteinuria at the time of biopsy. Serum creatinine, 24-h urinary protein, total number of glomeruli, number of sclerosed glomeruli, and the Oxford MEST-C scores were recorded for each of these cases. The glomeruli in 20 biopsies of acute tubular injury with no comorbid conditions, chronicity, and ≥10 glomeruli were taken as controls.
All kidney tissue specimens were obtained by percutaneous needle biopsy. The tissue was routinely processed, embedded in paraffin, cut into 3-µm sections, and stained with hematoxylin–eosin, periodic acid-Schiff stain, Masson’s trichrome stain, and periodic acid-methenamine stain. The slides were scanned using the Philips IntelliSite Pathology Solution-Ultra Fast Scanner. The glomeruli were annotated digitally, and the mean and maximum (max) GD were calculated for the controls and test cases as per the guidelines by Kataoka et al.5 and Tsuboi et al.8 [Figures 1 and 2].
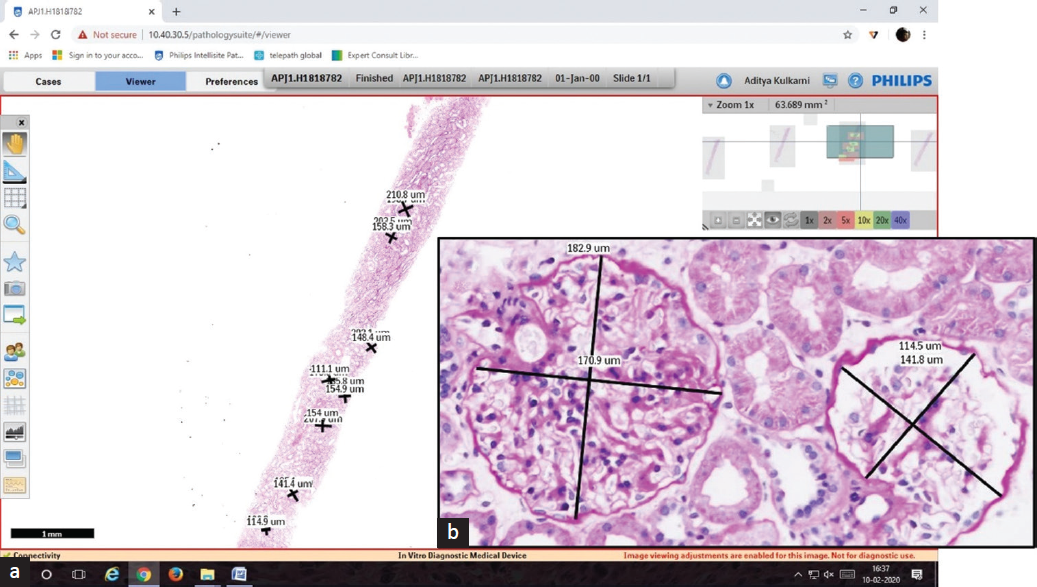
- (a and b) Whole slide imaging of a Periodic Acid Schiff stained section and digitally annotated glomeruli (a: H&E stain 0.5x, b: 40x). H&E: hematoxylin and eosin.
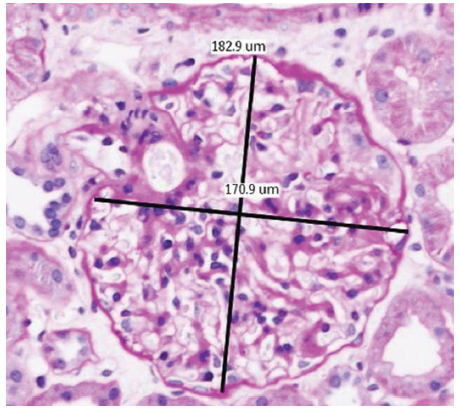
- Measurement of glomerular diameters (H&E stain, 20×). H&E: hematoxylin and eosin.
Calculation of the mean and maximum GD
The maximal diameter (which passes through the geometric center of the maximal profile of the glomerulus) and the maximal chord perpendicular to the maximal diameter were recorded in each viable glomerulus [Figure 2]. Mean GD was calculated as the mean of these two measurements. Max GD was taken as the mean of the maximally enlarged glomerulus.5 Sclerosed, ischemic glomeruli with shrinkage of the entire tuft and glomeruli with <40% area visible were excluded.8
The mean and max GD of the control and test biopsies were compared. The mean and max GD of the test cases were correlated with serum creatinine, 24-h urinary protein, and Oxford MEST-C scores.
Statistical analysis
The data was compiled and analyzed using MS Excel (R) office 365, GraphPad prism 8.4.2, and Statistical Package for the Social Sciences (SPSS) version 25. Descriptive statistics were presented in the form of proportions/percentages for categorical variables and mean and standard deviation for continuous data variables. Correlation of the parameters with GD values was done using the Spearman rho/Pearson R correlation coefficient (based on the normalcy and categorical/continuous nature of one or more of the independent variables). P-value of <0.05 was considered significant. A receiver operator characteristic (ROC) curve analysis was done to analyze the role of mean and max GD in the assessment of E scores.
Results
We included 100 patients of IgAN in this study, with a mean age of 34.67 years (11–64 years). Sixty-five of these patients were male and 35 were female, resulting in a male:female ratio of 1.8:1. The average serum creatinine for these patients was 1.82 mg/dl (0.44–9 mg/dl), and the average 24-h urinary protein was 4.01 g/day (0.13–54.90 g/day) [Table 1].
| Parameters | Observation | |
| Age in years | ||
| Mean ± SD | 34.67 ± 12.03 | |
| Range (Min–Max) | 53 (11–64) | |
| Gender | ||
| Male/female | 65/35 | |
| Creatinine levels (mg/dl) | ||
| Mean ± SD | 1.82 ± 1.62 | |
| Range (Min–Max) | 8.56 (0.44–9) | |
| Proteinuria | ||
| Nil/trace | 4 (4%) | |
| 1+ | 3 (3%) | |
| 2+ | 25 (25%) | |
| 3+ | 35 (35%) | |
| 4+ | 16 (16%) | |
| 24-h urinary protein (g/day) | ||
| Mean ± SD | 4.01 ± 6.30 | |
| Range (Min–Max) | 54.77 (0.13–54.90) | |
| Scores | ||
| M (0/1) | 34/66 | |
| E (0/1) | 57/43 | |
| S (0/1) | 42/58 | |
| T (0/1/2) | 82/15/3 | |
| C (1/2/3) | 57/33/10 | |
| Number of glomeruli | ||
| Mean ± SD | 15.47 ± 5.71 | |
| Range (Min–Max) | 28 (8–36) | |
| Mean glomerular diameter (µm) | ||
| Mean ± SD | 151.82 ± 28.69 | |
| Range (Min–Max) | 170.77 (99.9–270.67) | |
| Max glomerular diameter (m) | ||
| Mean ± SD | 205.40 ± 32.76 | |
| Range (Min–Max) | 157.20 (140.6–297.8) | |
SD: standard deviation; M: mesangial hypercellularity; E: endocapillary hypercellularity; S: segmental sclerosis; T: tubular atrophy; C: crescents.
The total number of glomeruli, number of sclerosed glomeruli, and the Oxford MEST-C scores were recorded for all the test cases on histopathology. On an average, the biopsies had 15.47 glomeruli (range 8–36 glomeruli). The average number of sclerosed glomeruli was 2.74 (24–0). The mean and max diameter were calculated for both the control and test cases. The average mean GD in the cohort of IgAN was 151.82 µm (99.9–270.67 µm), whereas the average max GD 205.40 µm (140.6–297.8 µm) [Table 1].
The mean GD was 156.96 µm in the control group compared to 151.83 µm in the test group. Similarly, the max GD was calculated to be 198.79 µm in the control group compared to 205.40 µm in the test group. The mean and max GD were similar for both the groups with no significant difference [Table 2].
| Mean GD | Controls | Cases | P-value |
| Number | 20 | 100 | 0.174 |
| Mean | 156.96 | 151.83 | |
| SD | 15.00 | 28.70 | |
| Max GD | Controls | Cases | P-value |
| Number | 20 | 100 | 0.5279 |
| Mean | 198.79 | 205.40 | |
| SD | 24.89 | 32.76 |
GD: glomerular diameter; SD: standard deviation.
We found no statistically significant correlation for the mean and max GDs with serum creatinine levels, 24-h urinary protein, Oxford MEST-C scores, and the number of sclerosed glomeruli [Table 3]. There was no significant difference between the mean and max GD in any Oxford MEST-C score categories (P > 0.05).
| Mean GD – correlation univariate analysis | |||||
| Parameters | Spearman rho | 95% CI | P-value | Significance | n |
| Age | 0.07 | −0.13 to 0.27 | 0.4882 | No | 100 |
| Creatinine | −0.054 | −0.25 to 0.15 | 0.5967 | No | 100 |
| 24-h urinary protein | −0.085 | −0.28 to 0.11 | 0.4023 | No | 100 |
| M | −0.0088 | −0.21 to 0.19 | 0.9309 | No | 100 |
| E | 0.2 | −0.0058 to 0.39 | 0.05 | No | 100 |
| S | 0.19 | −0.015 to 0.38 | 0.0619 | No | 100 |
| T | −0.11 | −0.31 to 0.090 | 0.258 | No | 100 |
| C | −0.032 | −0.23 to 0.17 | 0.7548 | No | 100 |
| Number of glomeruli | 0.12 | −0.087 to 0.31 | 0.246 | No | 100 |
| Number of sclerosed glomeruli | 0.04 | −0.16 to 0.23 | 0.6912 | No | 100 |
| Max GD – correlation univariate analysis | |||||
| Age | 0.035 | −0.17 to 0.24 | 0.7294 | No | 100 |
| Creatinine | 0.079 | −0.12 to 0.28 | 0.4335 | No | 100 |
| 24-h urinary protein | 0.015 | −0.18 to 0.21 | 0.8831 | No | 100 |
| M | −0.12 | −0.32 to 0.083 | 0.2303 | No | 100 |
| E | 0.17 | −0.032 to 0.36 | 0.0883 | No | 100 |
| S | 0.13 | −0.072 to 0.33 | 0.1906 | No | 100 |
| T | 0.035 | −0.17 to 0.24 | 0.7279 | No | 100 |
| C | −0.04 | −0.24 to 0.16 | 0.6933 | No | 100 |
| Number of glomeruli | 0.059 | −0.15 to 0.26 | 0.563 | No | 100 |
| Number of sclerosed glomeruli | 0.091 | −0.11 to 0.28 | 0.3678 | No | 100 |
CI: confidence interval; GD: glomerular diameter; M: mesangial hypercellularity; E: endocapillary hypercellularity; S: segmental sclerosis; T: tubular atrophy; C: crescents
ROC analysis of E score and mean/max GD
As the P-value of E scores approached significance levels, an ROC analysis was done to analyze the role of mean and max GD in the assessment of E scores [Figure 3]. This analysis revealed that the mean GD (P = 0.038) was more significant in predicting an E1 score compared to the max GD (P = 0.081) [Table 4]. Various cut-off values for mean GD were taken to calculate their sensitivity and specificity in predicting an E1 score. A lower cut-off value of 110 µm had a very high sensitivity (97%), whereas a much higher cut-off value of 180 µm had a higher specificity (92%). Therefore, a mid-range cut-off of 150 µm was evaluated, which had a fairly high sensitivity (61%) and specificity (62%) in predicting an E1 score [Table 5].
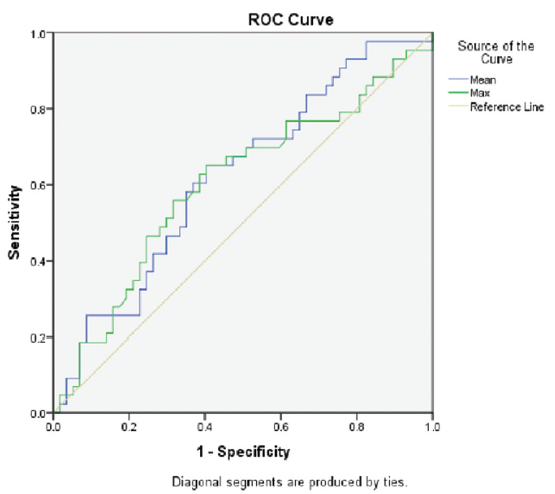
- ROC analysis for E score. ROC: receiver operating characteristic.
| Parameters for E score | AUC (Area under curve) | Significant |
| Mean GD | 0.621 | 0.038 |
| Max GD | 0.601 | 0.081 |
AUC: area under the ROC curve; GD: glomerular diameter: ROC: receiver operating characteristic; E: endocapillary hypercellularity.
| Cut-off value for mean GD (µm) | Sensitivity (%) | Specificity (%) |
| 110 | 97 | 8 |
| 150 | 61 | 62 |
| 180 | 18 | 92 |
GD: glomerular diameter, E: endocapillary hypercellularity.
Discussion
It has been well established by a great number of studies that glomerular hypertrophy plays crucial roles in the outcomes of kidney diseases.9 For example, the presence of glomerular hypertrophy in minimal change disease indicates progression to focal segmental glomerular sclerosis.10 Glomerular hypertrophy and renal hypertrophy are also seen soon after the onset of type 1 diabetic nephropathy.11 Persistent renal hypertrophy precedes the development of microalbuminuria and a decline in glomerular filtration rate (GFR) in early diabetic nephropathy.12 Thus, the presence of glomerular hypertrophy has implications with regard to susceptibility to glomerular sclerosis and renal insufficiency. Measurements of glomerular size have been found to be valuable to predict the progression of kidney diseases.13 However, we found a lack of literature regarding the use of this parameter in IgAN.
In this study, we attempted to establish a simple, reproducible, and quantitative histologic prognostic indicator in IgAN. In the earlier days, cumbersome techniques were necessary to assess the glomerular size. With the advent of whole slide imaging, fairly accurate GD measurements are now possible. However, age- and race-specific normal values need to be established before using them for disease measurement.14
Measurement of the max GD has its pros and cons. It includes the area occupied by the Bowman’s space and can be used to indicate the renal corpuscle size. This is a better measurement compared to the glomerular tuft size as it is less susceptible to sclerosis and collapse. Furthermore, the renal corpuscle is also easier to measure than the glomerular tuft.4 However, many problems exist regarding the measurement of GD. The size of each glomerulus varies over time. Therefore, glomeruli of varying sizes are seen within the same kidney as not all glomeruli are injured simultaneously. The area and the number of glomeruli sampled in a biopsy are limited and may or may not be an accurate representation of the entire kidney. The presence of ischemic, hypertrophied, or collapsed glomeruli can sometimes skew results, especially in a biopsy with fewer glomeruli. There is no consensus on whether to include these in the measurements. Lastly, there may be more than one disease process occurring simultaneously in a particular kidney, which may influence the GD measurements and thus should be accounted for.5,14
The pathophysiology of glomerular hypertrophy is still unclear, and there are few studies in literature which have tried to explain the same. Cortes et al.15 demonstrated the elasticity of glomerular capillaries and enlarged glomerular volume caused by increase in intraglomerular pressure, measured in rat focal segmental glomerulosclerosis.16,17 Another mechanism for glomerular hypertrophy has been described by Bohle et al.18 and Mackensen-Haen,et al.19 They observed that interstitial expansion due to fibrosis destroys the tight structural and functional connection between the tubules and peritubular capillaries. The obliteration of these capillaries leads to an increase in the resistance of the post-glomerular capillary network and, therefore, to a rise in the intraglomerular pressure, resulting in glomerular hypertrophy.16,20,21
Ours was a retrospective study consisting of 100 cases diagnosed as IgAN. We thus collected the clinical and histologic data of these cases and correlated it with their mean and max GD. Most patients were between 11 and 64 years, with the mean age being 34.67 years. Kataoka et al.5 and Tsuboi et al.8 also found the mean age range to be 40 and 30.6 years, respectively. While we found a male preponderance which was similar to the findings of Kataoka et al.,5 Tsuboi et al.8 reported a female preponderance. However, in contrast to their studies, we observed a higher mean value of 24-h proteinuria (4.01 g) and serum creatinine (1.82 mg/dl). For this, we propose the following reasons. Nephrotic syndrome has been reported as the main mode of clinical presentation in the Asian population.22 Screening programs in other countries detect patients at an earlier stage of the disease. The average value of max GD in our study was 205.4 µm (range 140.6–297.8 µm), which is close to the value reported by Kataoka et al.4 (221.7 µm). Tsuboi et al.8 recorded GD and glomerular area in their study.
In our study, we found no correlation of max as well as mean GD with the 24-h urinary protein, serum creatinine, number of sclerosed glomeruli, and the Oxford scores at the time of biopsy. Similar to our study, Kataoka et al.5 also did not find any correlation with the 24-h proteinuria at the time of biopsy. In their study, the max GD showed a significant positive correlation with age and body mass index (BMI), but not with proteinuria. However, at a 10-year follow-up, a significant correlation was established. They found that glomerular hypertrophy to a max GD ≥242 µm was associated with follow-up proteinuria and a 1.5-fold increase in serum creatinine values. They also felt that since max GD was found to be significantly correlated with age, BMI, and the level of serum triglyceride in their study, it could have the possibility to detect lifestyle insults. On dividing their patients into two groups (Group A ≥242 µm and Group B <242 µm), they found no significant difference in mesangial cell proliferation, endocapillary hypercellularity, extracapillary cellular proliferation, global glomerulosclerosis, and segmental glomerulosclerosis between the two groups. In another study by the same authors, they proposed that adding maximum GD to the Oxford MEST-C scores could significantly improve its prognostic ability.4 Tsuboi et al.,8 in a similar study, measured the mean glomerular area and the glomerular density in patients with IgAN. They also did not find any correlation with proteinuria or the percentage of sclerosed glomeruli on that biopsy. The extent of acute lesions such as cellular or fibrocellular crescent formation, mesangial proliferation, and intracapillary proliferation also showed no such correlations. However, while glomerular density at the first biopsy significantly correlated with change in creatinine clearance, mean glomerular area did not show any such correlation. Tóth and Takebayashi16 did a morphometric analysis of glomerular capillary loop size in 52 pediatric patients with IgAN. They found that it was significantly greater in patients who had chronic renal failure when compared to controls or the ones who did not have chronic renal failure. The 10-year renal survival rates of patients with a “large” loop size (11.55-fold) were also significantly lower than those of patients with a smaller capillary loop size.
Our study had a few limitations. Follow-up biopsies were not taken to assess the utility of GD in progression of disease. Other renal disorders affecting GD, such as obesity and hypertension, were not completely excluded.
Conclusion
The mean and max GD did not correlate with serum creatinine, 24-h urinary protein, and Oxford MEST-C scores at the time of biopsy in our study. We found that at a cut-off value of 150 µm for mean GD, there is a 61% sensitivity and 62% specificity in predicting E1 score. Thus, GD is a parameter that can now be objectively and accurately measured and may be of significance. However, further studies with follow-up are needed to determine its utility in IgAN.
Acknowledgements
The authors would like to thank the technical team of Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, India for providing them with good-quality sections and special stains.
Conflicts of interest
There are no conflicts of interest.
References
- Histological subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829-42.
- [CrossRef] [PubMed] [Google Scholar]
- Histological grading of IgA nephropathy predicting renal outcome: Revisiting HS Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342-8.
- [CrossRef] [PubMed] [Google Scholar]
- Maximum glomerular diameter and oxford MEST-C score in IgA nephropathy: The significance of time-Series changes in pseudo-R2 values in relation to renal outcomes. J Clin Med. 2019;8:2105.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol Dial Transplant. 2011;26:3937-43.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction to digital image analysis in whole-slide imaging: A white paper from the digital pathology association. J Pathol Inform. 2019;10:9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249:F324-37.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in the glomerular density and size in serial renal biopsies during the progression of IgA nephropathy. Nephrol Dial Transplant. 2009;24:892-9.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerular hypertrophy accelerates hypertensive glomerular injury in rats. Am J Physiol. 1991;261:459-65.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115-23.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia. 1975;11:225-9.
- [CrossRef] [PubMed] [Google Scholar]
- Global heterogeneity of glomerular volume distribution in early diabetic nephropathy. Kidney Int. 2004;66:855-61.
- [CrossRef] [PubMed] [Google Scholar]
- Applicability of the glomerular size distribution coefficient in assessing human glomerular volume: The Weibel and Gomez method revisited. J Anat. 2007;210:578-58.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerular number and size: Facts and artefacts. Anat Rec. 1998;251:66-71.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerular hypertension and progressive renal disease: The interplay of mesangial cell stretch, cytokine formation and extracellular matrix synthesis. In: Koide H, Ichikawa I, eds. Progression of Chronic Renal Diseases. Vol Vol. 118. Contrib Nephrol. Basel: Karger; 1996. p. :229-33.
- [Google Scholar]
- Glomerular hypertrophy as a prognostic marker in childhood IgA nephropathy. Nephron. 1998;80:285-91.
- [CrossRef] [PubMed] [Google Scholar]
- Renal functional adaptation in the remnant kidney in patients with renal agenesis and in patients nephrectomized in childhood. Acta Pediatr Scand. 1978;67:611-5.
- [CrossRef] [PubMed] [Google Scholar]
- The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubules. Functional interpretation of morphologic findings. Klin Wochenschr. 1981;59:1043-51.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution on the correlation between morphometric parameters gained from the renal cortex and renal function in IgA nephritis. Lab Invest. 1988;59:239-44.
- [PubMed] [Google Scholar]
- Morphometric analysis of the glomerular capillary area – A comparison of minimal change nephrotic syndrome, focal glomerular sclerosis, and pre-eclampsia. J Pathol. 1991;165:329-36.
- [CrossRef] [PubMed] [Google Scholar]
- The progression of renal disease: Structural and functional correlation. In: Tischer CC, Brenner BM, eds. Renal Pathology. Vol Vol. 1. Philadelphia: Lippincott; 1994. p. :116-39.
- [Google Scholar]
- IgA nephropathy in a tertiary care center from south India. Indian J Nephrol. 2011;21:230-4.
- [CrossRef] [PubMed] [Google Scholar]







