Translate this page into:
His Heart Broke Posttransplant: A Rare Disease with Good Outcome
Address for correspondence: Dr. Bhanu Mishra, 16E, Nivedita Enclave, Block A6, Paschim Vihar, New Delhi - 110 063, India. E-mail: bhanumishra.1289@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Sir,
In a given case of end-stage renal disease (ESRD), renal transplantation is the most preferred and definitive modality of treatment. Out of many post transplant complications, infection, and rejection are the most common. Here, we describe a rare case of Takotsubo Cardiomyopathy Syndrome (TCM), which has hardly been reported, especially in a live-related renal transplant on postoperative day 2.
A 58-year-old male, ESRD due to focal segmental glomerulosclerosis (FSGS), underwent live donor renal transplant with his wife being the donor. All necessary clearances were obtained and his baseline electrocardiogram, two-dimensional (2D) echocardiogram (LVEF 55%), and dobutamine stress echocardiogram were normal. His surgical course was uneventful. He was given antithymocyte globulin (ATG) induction (75 mg, three doses) and was managed on triple immunosuppression with tacrolimus (0.1 mg/kg/day), mycophenolate sodium (720 mg/day), and steroids (as per protocol). He had brisk urine output and creatinine showed downward trend. Forty-eight hours postoperatively, he complained of chest pain, breathing difficulty, and within hours developed severe pulmonary edema. His electrocardiogram showed sinus tachycardia with non-significant (<2 mm) ST-T depression in precordial leads (v1–v4). Cardiac enzymes (hsTrop I) were high (196.9pg/ml) and serial monitoring showed increasing trend (2636.1pg/ml). 2D echocardiogram showed apical ballooning with low ejection-fraction of 30% with increased pulmonary arterial pressure (PASP 60 mm hg) [Figure 1]. Cardiology team advised urgent coronary angiography (CAG). CAG revealed non-obstructive coronary flow with no plaque or focal vasospasm with Thrombolysis In Myocardial Infarction (TIMI) score being 3. The patient became hypotensive requiring inotropic support (noradrenaline 0.1 μg/kg/min). His blood pressure continued to be low and in view of falling saturation, he was intubated and given ventilatory support (Pressure Regulated Volume Control mode, Fraction of inspired O260%, and Positive End Expiratory Pressure 8). Intra-aortic balloon counter pulsation (IABP) was initiated to augment the cardiac function. Patient gradually started showing signs of improvement with gradual tapering inotropic support and weaning of ventilator after 24 h. On day 4, he was extubated, IABP stopped, and withdrawn. All along patient maintained good urine output with stable graft function. His tacrolimus level was 6.4 ng/mL and tacrolimus dose was adjusted to maintain levels of 10–12 ng/mL. On day 6, he was discharged with creatinine of 1.3 mg/dL and is currently following up regularly in out-patient service with good cardiac function (LV EF 55%) and graft function.
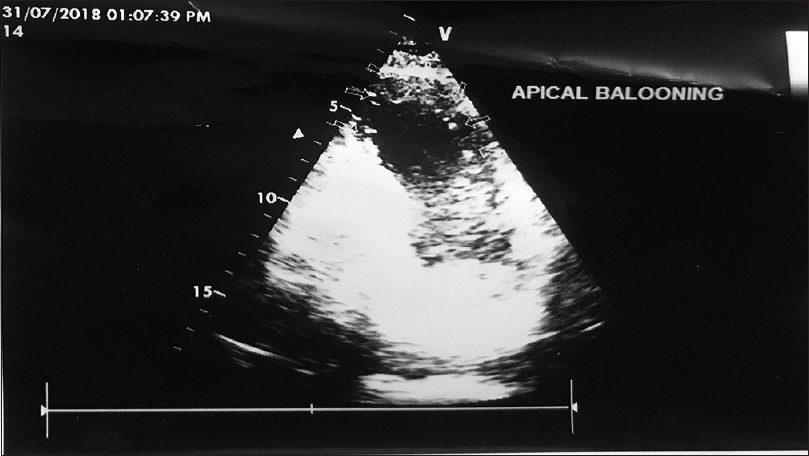
- Dual chamber view-apical ballooning
TCM is a form of acute reversible left ventricular dysfunction characterized by apical-ballooning of a hypo or akinetic left ventricular apex with hyperdynamic basal walls. TCM is also known as transient left ventricular apical ballooning syndrome, broken heart syndrome, and stress cardiomyopathy. In most cases, an acute emotional or physical stressor, such as surgery, trauma, grief, etc., can be identified as a triggering factor.[1]
Based on Mayo Clinic Criteria, TCM is commonly defined by the following points: (a) transient hypokinesis, akinesis, or dyskinesis in the left ventricular mid segments with or without apical involvement, and frequently, but not always, a stressful trigger; (b) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (c) new ECG abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (d) absence of pheochromocytoma and myocarditis.[2] Coronary microvascular dysfunction, coronary artery spasm, catecholamine-induced myocardial stunning, reperfusion injury following acute coronary insufficiency, myocardial micro-infarction, and abnormalities in cardiac fatty acid metabolism have all been suggested as possible pathophysiology mechanisms.[1]
In our patient, we ruled out myocardial infarction as echocardiogram was typical of TCM and coronary angiography was normal. Myocarditis and pulmonary embolism being the other differential were ruled out in view of the normal biochemical and electrocardiographic and echocardiographic findings (Right Ventricle function normal). There have been several reports of TCM in patients undergoing solid organ transplantation and first case post renal transplant was reported by Chrapko et al.[3] As in our case, in the previously reported cases, TCM was apparent in initial 48 h after renal transplant. Tacrolimus toxicity was considered the causal agent in the case reported by Gołebiewska et al.[4] tacrolimus levels being 40.5 ng/mL, but in our case, tacrolimus levels were 6.4 ng/mL. In case of report given by Vailas et al.,[5] the potential role of exogenous administration of norepinephrine was thought as the causal agent as they had to use the same for intraoperative hypotension, but in our case surgery was uneventful.
In conclusion, to the best of our knowledge, this is the fourth reported case of TCM following renal transplantation and first from India. In our case, there was no evidence of exogenous noradrenaline exposure or tacrolimus toxicity. The severe stress reaction of the patient along with rising troponin, decreased left ventricular ejection fraction, ballooning of the apex with normal coronaries and complete recovery of the cardiac function later were strongly suggestive of the diagnosis of TCM. This is a rare case and may help others in the same situation. The important message is that sudden cardiac deterioration in the postoperative period should be aggressively managed and can bring good outcomes in few patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Takotsubo syndrome in a patient after renal transplantation. Med Sci Monit. 2012;18:CS 26-30.
- [Google Scholar]
- Tako-tsubo cardiomyopathy on the first day after renal transplantation - Case report and Literature review. Transplant Proc. 2014;46:2920-2.
- [Google Scholar]
- A heartbreaking renal transplantation: Is norepinephrine the culprit to blame. Transplant Proc. 2016;48:3088-91.
- [Google Scholar]






