Translate this page into:
Hypothyroidism Related Kidney Disease - A Report of Two Cases
Corresponding author: Ajay Jaryal, Department of Medicine, AIIMS, Bilaspur, Himachal Pradesh, India. E-mail: drajayjaryal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jaryal A, Vikrant S, Sharma A. Hypothyroidism Related Kidney Disease - A Report of Two Cases. Indian J Nephrol. doi: 10.25259/IJN_266_2024
Abstract
Dysthyroid state affects kidneys in multiple ways. Hypothyroidism has been described as a cause of renal dysfunction in case reports, and a few large-scale epidemiological studies have shown association of hypothyroidism with abnormalities of renal function parameters. Restoration and improvement in renal functions have also been reported with treatment of hypothyroidism. We highlight two cases where hypothyroidism contributed to renal dysfunction and the treatment of hypothyroidism led to improvement in renal function.
Keywords
Hypothyroidism
Acute kidney injury
Chronic kidney disease
Introduction
Kidneys are predisposed to dysfunction because of systemic causes and hypothyroidism can be one of those. Hypothyroidism is most common endocrine disorders in patients of chronic kidney disease (CKD) and contributes to development of acute kidney injury (AKI) and CKD.1 Treatment of hypothyroidism can partially or wholly mitigate renal dysfunction. Our current case series highlights hypothyroidism contributing to AKI and CKD, and treatment of hypothyroidism led to complete resolution of AKI and improved estimated glomerular filtration rate (eGFR) in a patient of CKD.
Case Reports
Case 1
A 65-year-old female presented with complaints of swelling feet for 2 months, a gradual fall in urine output for 3 weeks and anuria for 1 day. The examination showed anasarca, hoarseness of voice, and hypertension. Investigation revealed hemoglobin (HB) 13.2 g/dL, TLC 7320/µL, platelet count 4.2 lakh/µL, urea 276 mg/dL, creatinine 10.6 mg/dL, uric acid 7.8 mg/dL, sodium 129 mEq/L, potassium 4.1 mEq/L, normal liver function tests, corrected calcium 8.8 mg/dL, phosphorous 11.6 mg/dL, cholesterol 195 mg/dL, triglyceride 275 mg/dL, protein 5.4 g/dl, albumin 0.9 g/dL, urine: protein 3 + RBC 4-5/hpf WBC 4-5/hpf, and normal USG of kidneys. She was started on hemodialysis (HD) and after 3 sessions of HD a kidney biopsy was done. Her further investigations revealed normal complement, T4 2.4 µg/dL (3.2–12.6), TSH 30 mIU/mL (0.35–4.5), ANA 1 + speckled, CPK 110 U/L (30–170), LDH 499 U/L (208–378), anti-TPO antibodies 222.8 U/L (< 5.34). L-thyroxine was started for primary hypothyroidism. Kidney biopsy revealed FSGS and pigmented granular cast, which stained positively for myoglobin [Figure 1]. So, our patient most likely had spontaneous rhabdomyolysis due to hypothyroidism leading to myoglobin cast nephropathy. We couldn’t do workup for myoglobinuria as there was no clinical lead to suspect it initially, and by the time renal biopsy report came, it was quite late. Methylprednisolone followed by oral steroids were added in view of primary FSGS. Patient was discharged from the hospital after return of S. creatinine in the normal range. On follow-up at 3 months, her investigation showed: S. creatinine 0.9 mg/dL, albumin 3.6 g/dL and 24-hour urine protein 450 mg. In our patient AKI, was most likely orchestrated by combination of hypothyroidism related myoglobin cast nephropathy and nephrotic syndrome.
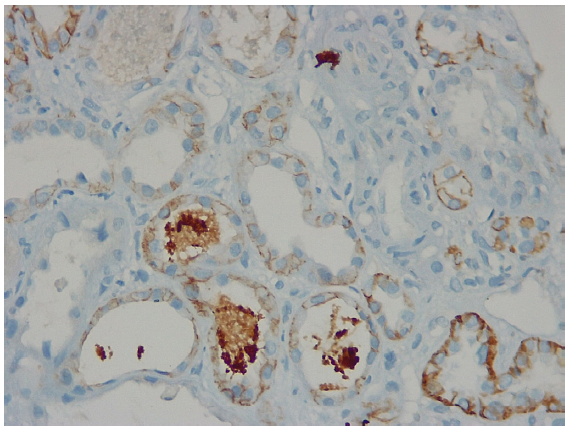
- Kidney biopsy showing pigmented granular cast staining positively for myoglobin and negatively for hemoglobin.
Case 2
A 79-year-old normotensive and non-diabetic male with history of elevated serum creatinine for one year and then lost to follow up, presented to the outpatient clinic. On examination, he had periorbital edema, hoarseness of voice, coarse skin, slowness of activities and delayed relaxation of ankle jerks. His main investigations are tabulated in Table 1. He had a normal renal ultrasound, CPK 162 U/L and anti TPO antibodies 130 U/L and was started on escalating doses of L thyroxine and on follow up 3 months later, his serum creatinine, proteinuria and thyroid hormone levels have improved. His eGFR (CKD EPI) improved from 35 to 49 mL/min/1.73 m2 after restoration of euthyroid state. This indicates that hypothyroidism might be a potential reversible factor exacerbating kidney function decline in individuals with other CKD causes or as a standalone cause of CKD.
| Month | HB | Creatinine | AST | Cholesterol | Triglyceride | T4 | TSH | Urine protein |
|---|---|---|---|---|---|---|---|---|
| 0 | 8.6 | 2.02 | 58 | 195 | 172 | 0.3 | >150 | 2+ |
| 3 | 10.4 | 1.5 | 19 | 70 | 71 | 8.4 | 1.45 | trace |
HB: Hemoglobin, AST: Aspartate aminotransferase, T4: Tetraiodothyronine, TSH: Thyroid stimulating hormone.
Discussion
Thyroid hormone has an important role in kidney structure and function both during growth and development, and in adulthood.1 In experimental models, hypothyroidism leads to alteration in structure and function of various substructures of kidney and reduced kidney-to-body weight ratio.2 In a large cross-sectional study amongst individuals ≥ 55 years, it was observed that those with hypothyroidism were more likely to have CKD. Hypothyroidism may lead to kidney dysfunction via effects of thyroid hormones on cardiac output, metabolism, intra-renal hemodynamics, and renin angiotensin aldosterone system (RAAS), as well as structural changes.3 Treatment of hypothyroidism by thyroid hormones in patients with renal dysfunction has been demonstrated to be effective in improving renal functions.4 Hypothyroidism can also lead to AKI by rhabdomyolysis or even without rhabdomyolysis. In the second scenario, kidney biopsy changes have been described which could be characteristic of hypothyroidism, as marked regression of kidney biopsy changes has also been demonstrated after restoration of euthyroidism in few patients.5,6 Rhabdomyolysis usually occurs in the presence of some precipitating factor like drugs or strenuous exercise but rarely spontaneous rhabdomyolysis can also occur in hypothyroidism.7 Treatment of hypothyroidism not only improves renal function but also has favorable effects on dyslipidemia and cardiorenal interactions improving overall cardiovascular and renal risk profile. So, screening for thyroid function should be made an integral part of evaluation of AKI and CKD and euthyroidism should be restored as permitted by the underlying cardiovascular status.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- The interplay between thyroid dysfunction and kidney disease. Semin Nephrol. 2021;41:133-43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Thyroid-induced changes in the growth of the liver, kidney, and diaphragm of neonatal rats. J Cell Physiol. 1994;161:49-54.
- [CrossRef] [PubMed] [Google Scholar]
- Association between hypothyroidism and chronic kidney disease observed among an adult population 55 years and older. Medicine (Baltimore). 2020;99:e19569.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hypothyroidism and reversible kidney dysfunction: An essential relationship to recognize. Endocr Pract. 2014;20:490-9.
- [CrossRef] [PubMed] [Google Scholar]
- Renal lesions in hypothyroidism: A study based on kidney biopsies. Metabolism. 1967;16:846-52.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury and hypothyroidism in a patient with CKD. Kidney Dialysis. 2022;2:537-44.
- [Google Scholar]
- Rhabdomyolysis in a patient with severe hypothyroidism. Am J Case Rep. 2017;18:912-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







