Translate this page into:
Imlifidase: Is it the Magic Wand in Renal Transplantation?
Corresponding author: Nithya Krishnan, Department of Renal and Transplant Medicine, Institute of Cardiometabolic Medicine, University Hospitals Coventry and Warwickshire NHS Trust, Coventry, United Kingdom. E-mail: Nithya.krishnan@uhcw.nhs.uk
-
Received: ,
Accepted: ,
How to cite this article: Krishnan N, Briggs D. Imlifidase: Is it the Magic Wand in Renal Transplantation? Indian J Nephrol. 2024;34:291-6. doi: 10.25259/ijn_325_23
Abstract
Potential kidney transplant patients with HLA-specific antibodies have reduced access to transplantation. Their harmful effects are mediated by the Fc portion of IgG, including activation of the complement system and Fc receptor-initiated cytotoxic processes by circulating leucocytes. Avoiding antibody incompatibility is the conventional approach, but for some patients this can mean extended waiting times, or even no chance of a transplant if there are no alternative, compatible donors. For these cases, pretransplant antibody removal may provide access to transplantation. Plasmapheresis is currently used to achieve this, with acceptable outcome results, but the process can take days to reduce the antibody levels to a safe level, so has limited use for deceased donors. There is now an alternative, in the form of an IgG-digesting enzyme, Imlifidase, which can be administered for in vivo IgG inactivation. Imlifidase cleaves human IgG, separating the antigen-binding part, F(ab’)2 from Fc. Typically, within six hours of dosing, most, if not all, of the circulating IgG has been inactivated, allowing safe transplantation from a previously incompatible donor. For deceased donor transplantation, where minimizing cold ischaemia is critical, this six-hour delay before implantation should be manageable, with the compatibility testing processes adjusted to accommodate the treatment. This agent has been used successfully in phase 2 clinical trials, with good short to medium term outcomes. While a donation rate that matches demand may be one essential answer to providing universal access to kidney transplantation, this is currently unrealistic. IgG inactivation, using Imlifidase, is, however, a realistic and proven alternative.
Keywords
Kidney transplantation
Imlifidase
HLA comparability
Sensitisation
Immunosuppression
Introduction
It is well established that Human Leucocyte Antigen (HLA) antibodies are a barrier to successful transplantation. Transplantation, where the potential recipient carries donor-specific antibodies (DSAs), should usually be avoided to prevent immediate or early graft loss.1 The antibody-binding domain (Fab) of the DSAs binds to the mismatched HLA antigens on the allograft. While this interaction can directly initiate changes to donor tissue, which can be pro-survival,2 the harmful effects are mediated by the Fc portion of the antibody, including regulation of immune cells and complement- and cell-dependent cytotoxicity. The resulting damage can lead to early graft loss.
HLA antibodies are formed following previous sensitization from a blood transfusion, previous transplantation, or pregnancy.3 The possibility of sensitization escalates if there is more than one sensitizing risk factor.4 The number of DSAs in a recipient against potential donors can be measured by the calculated reaction frequency (CRF) in the UK. This is an estimate of the probability of an allocated deceased donor being antibody incompatible by comparing the recipient’s antibody profile with the HLA types of the UK’s last 10,000 deceased donors. The higher the CRF percentage, the lower the chance of being offered a compatible kidney.5 Other countries use panel reactivity antibody (PRA) or calculated PRA (cPRA), which are similar measures. PRA is a percentage of the local pool of organ donors to which a patient has reactive antibodies.1 A patient with 80% PRA would be crossmatched and incompatible with 80% of donors. The calculated CPRA is based upon unacceptable HLA antigens to which the patient has been sensitized and which, if present in a donor, would represent an unacceptable risk for the candidate or the transplant program.6 The higher the CPRA, the fewer offers would be received.
Studies have shown that between 10% and 30% of patients who await renal transplantation are sensitized, both in India and worldwide.7,8 Avoidance of incompatibility, that is, not transplanting across a positive crossmatch, is the standard approach to transplanting HLA-sensitized potential recipients. This typically results in long waiting times, which compromises overall patient survival and quality of life. The alternative is to remove the problem. Pretransplant antibody removal or reduction is feasible in certain cases using techniques such as plasmapheresis (PP), and this has been applied since the mid-1980s.9 However, this usually requires successive rounds of treatment over a few days, and very high DSA levels can be difficult to reduce to a safe transplantation level. Recipients with larger amounts of preformed DSAs have a higher risk of graft loss.10,11 Despite the increased immunological risks, transplanting against specific immunological memory and the probability of DSA resynthesis, long-term outcomes of such HLA incompatible transplants can be good, and in our experience equivalent to the outcomes seen for first-time, deceased donor transplants in the UK.12 The overall patient survival of antibody incompatible transplantation (AIT) was 95%, 89%, and 81%, and graft survival was 95%, 85%, and 70% at 1, 5, and 10 year, respectively, is similar to observations in a multi-center study reported by Orandi et al.,13 which showed that the patient survival of the highly sensitized patients if transplanted was 77% at 8 years post-transplant.
However, for recipients who do not have a live donor, the constraints of factors such as cold ischemia time mean that conventional pretransplant antibody removal is usually not possible with deceased donor transplantation. Therefore, patients with a high CRF wait for a deceased donor kidney significantly longer than non-sensitized patients. The UK Renal Registry annual report 2018 showed that 10-year survival of all patients between the age groups of 18 and 64 years on renal replacement therapy (which includes dialysis and transplants) was 55%. To improve the survival outcome of highly sensitized patients on the transplant waiting list who do not have a live donor, antibody removal or inactivation at the time of a donor offer might provide their only route to transplantation. Rapid in vivo antibody inactivation can be achieved following administration of the recombinant cysteine protease, Imlifidase, derived from S. pyogenes, and this has been used successfully in recent trials, demonstrating safety and effectiveness.14,15
Imlifidase, the drug
Imlifidase is a recombinant cysteine protease that specifically degrades human IgG antibodies16 although the IgG from certain other species also show sensitivity to degradation. The enzyme, previously referred to as IdeS, cleaves IgG at the lower hinge region to form F(ab’)2 and Fc fragments. Initially, within minutes of administration, circulating IgG is digested to give single-cleaved IgG (scIgG), where just one of the two IgG heavy chains is cut. This is followed within a few hours by cleavage of the second heavy chain in the molecule, producing one F(ab’)2 and one homodimeric Fc fragment [Figure 1]. After this, there can be no intact IgG and only low levels of scIgG remaining.14 Other human proteins, including immunoglobulins IgM, IgA, IgD, and IgE, are not degraded by Imlifidase, but all four human subclasses of IgG are sensitive to Imlifidase. Full IgG cleavage leads to the inactivation of all IgG-dependent Fc-dependent effector functions, including antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis,17 and complement-dependent cytotoxicity (CDC).18 However, F(ab)2 activities, such as intracellular signal-mediated receptor cross-linking or virus neutralization due to receptor domain binding (RBD), should be preserved. RBD-specific F(ab)2 fragments, for example, have potent SARS-Cov-2 neutralization activity.19 The phase 1 study by Winstedt et al.14 showed that Imlifidase has an in vivo half-life of about 4.9 hours, and by 24 hours, the main fraction will mostly have been eliminated. The lowest levels of detectable, intact IgG were seen between 6 and 24 hours post-dosing.
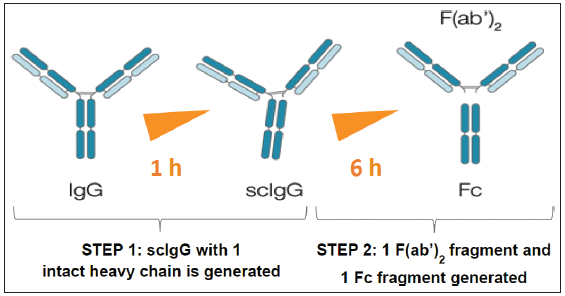
- Imlifidase, an immunomodulatory streptococcal protease agent, cleaves IgG in a 2-step process (Winstedt L, et al. PLOS ONE. 2015;10(7):e0132011).
Imlifidase, clinical uses
The first report of clinical utility in transplantation was obtained from the combined experience of two independently performed, open-label, phase 1–2 trials conducted in Sweden and the United States, that IdeS reduced or eliminated DSAs and permitted HLA-incompatible transplantation in 24 of 25 patients.20,21 This shows the value of Imlifidase in providing a window of transplant opportunity using an antibody-incompatible donor. The time from starting the treatment to engraftment should be measured in hours rather than the days typically required for PP-based desensitization. The situation where Imlifidase is inferior to PP is if the DSA is an IgM and most likely to be non-HLA specific. For this reason, Imlifidase is not indicated to enable ABO-incompatible transplantation.
As Imlifidase allows acute elimination of IgG-mediated effects, this agent might have a role in post-transplant management.22 This would require circumstances where there is clear evidence of significant IgG-mediated graft damage involving high levels of DSA. Diagnosed AMR can be effectively treated with conventional immunosuppression agents and the DSA rebounds seen after HLA AIT very often spontaneously modulate,23 and this is also seen following Imlifidase desensitization.20 The post-transplant use of Imlifidase needs to be subject to clinical trial. There is a case report of Imlifidase being used in the treatment of acute AMR of a liver transplant,24 but the contribution of Imlifidase, in this case, is masked by the use of other agents, and the levels of the DSAs had declined significantly before Imlifidase was administered. A study is now underway for the treatment of AMR (NCT03897205) that compares Imlifidase versus PP among kidney transplant patients with acute or chronic active AMR.
Clinical trials to date
Jordan et al.20 published in 2017 the output from two independent phases 1–2 studies involving 25 patients, 11 patients in Sweden and 14 in the US, who had an HLA incompatible transplants following Imlifidase injection: 22 had DSAs, 18 of whom had a positive flow cytometry cross-match, and two were CDC crossmatch-positive. The patients were given Imlifidase 4–6 hours before transplant, followed by horse anti-thymocyte globulin (ATG) in the Swedish cohort and alemtuzumab in the US cohort. Patients in the United States were treated with IVIG 2 g/kg on days 7–14 and rituximab 375 mg/m2 on days 14–21 after transplant. 24 of 25 patients had functioning allografts after transplantation, and AMR occurred in 7 patients in the U.S. and 3 in the Swedish group between 2 weeks to 5 months after transplantation. One graft was lost, likely due to non-HLA IgM and IgA antibodies, but the other patients had a good response to treatment.
The Highdes trial was a phase 2 study25 that enrolled 19 patients from the US, Sweden, and France who had an incompatible living or deceased transplantation with a positive crossmatch. Thirteen patients received kidneys from deceased donors, and five patients received from live donors. DSAs greater than 3000 MFI were present in all patients, with a median cPRA of 99.83% (range: 77.31–100.0%). Imlifidase 0.25 mg/kg was given before the transplant, with an additional 0.25 mg/kg dose allowed if a negative cross-match was not achieved after the first dose. Patients had horse ATG or alemtuzumab as induction, IVIG 2 g/kg on posttransplant day 7, and rituximab 1 gram on day 9. Of 19 patients enrolled, 18 underwent transplantation. At 6 months, patient survival was 100%, and graft survival was 89%. Of the transplanted patients, 89.5% demonstrated conversion of baseline positive crossmatch to negative within 24 hours after Imlifidase treatment. DSAs bounced back 3 to 14 days post-Imlifidase dose, with substantial interpatient variability. Patient survival was 100%, with graft survival of 88.9% at 6 months. With this, 38.9% had early biopsy-proven antibody-mediated rejection with onset 2–19 days post-transplantation. Serum IgG levels began to normalize after 3–7 days post-transplantation.
In a pooled study of four open-label single-arm, phase 2 clinical trials, long-term outcomes of 39 highly-HLA sensitized (median cPRA 99.62%) and crossmatch-positive patients transplanted after desensitization with imlifidase therapy were reported.26 The incidence of AMR was 38%. Among patients who experienced AMR, the MFI of the immunodominant DSA pre-imlifidase treatment was significantly higher (median MFI ∼13,000, IQR 6500–22,000) compared to those who did not develop AMR (median MFI ∼6000, IQR 3000–9000; P < .05). At three years, patient survival was 90%, allograft survival was 84%, and the mean eGFR was 55 ml/min/1.73 m2. A subgroup analysis of deceased donor recipients with cPRA ≥99.9% considered unlikely to be transplanted without imlifidase desensitization exhibited similar graft survival and eGFR to the overall population but a higher AMR rate. These data suggest that imlifidase desensitization could be useful for the most highly HLA sensitized patients unlikely to receive a transplant under the current KAS.
Antibody-mediated rejection
Although there is no comparator arm in the available clinical data, many AIT transplants done post-PP in expert centers worldwide have shown an early antibody-mediated rejection (AMR) rate in the range of 40%.12,27,28 Therefore, the AMR rate of 40% with Imlifidase treatment is to be expected. However, despite the high rate of AMR, the long-term outcomes of AIT are very good.12,13
Patients to be considered for imlifidase
In the UK, approval has been given for the use of a single dose of Imlifidase to enable deceased donor transplantation in highly sensitized waiting list patients.29 Acceptance criteria limit access to those with cRF of 99% or greater a matchability score30 of 10 and on the waiting list for more than 2 years, which seems a reasonable starting point for consideration of Imlifidase in this group of patients. It may be desirable that the criteria of inclusion could be expanded to other long waiters with cRFs of >95% and perhaps even include live donor transplantation once the success of Imlifidase is demonstrated in this group of individuals. While PP is the standard mode of desensitization with live donors, there is an increased risk of bleeding intra-operatively due to the concomitant removal of coagulation proteins during the process. In addition, there is an increased risk of severe hypotension with the associated risk of morbidity like blindness and mortality, which precludes many patients from undergoing PP and hence the opportunity to have a transplant. Finally, high levels of antibodies and certain types of HLA antibodies are not easily removed by PP. Thus, an intervention like Imlifidase would be the best option for transplantation for such patients, whether from live or deceased donors.
With deceased donor transplantation, the exact nature of any incompatibility can only be known once a potential donor has been HLA typed and possibly crossmatched. Each candidate patient’s antibody profile would have to be reviewed, and a list of acceptable incompatible HLA mismatches would be determined. Removing these from the unacceptable antigen list should be designed to reduce the CRF to an extent that gives the patient a reasonable chance of a donor offer. This needs to be balanced with the outcome risks deemed acceptable to the patient. Without the sufficient experience of clinical Imlifidase use to provide evidence to support such risk assessment, it is reasonable to base these on the experiences from PP-based AIT. Factors including antibody strength, complement activation activity, and the number of donor-reactive specificities have all been shown to predispose to early rejection risk and reduced graft life.12,31–33
The use of Imlifidase is likely to require additional pretransplant compatibility testing with the potential to significantly increase cold ischemia times (CIT) with the risk of donor kidneys becoming unusable. There, therefore, needs to be a clear process agreed with all the participating agencies, from the donor coordinators, retrieval teams, and testing laboratories to the transplant surgeons and nephrologists, as suggested in Figure 2.
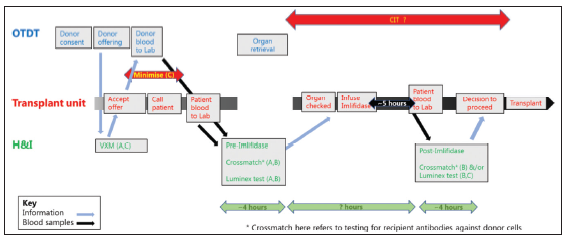
- Use of Imlifidase for an antibody incompatible deceased donor transplant. Suggested pathway to minimize cold ischemia time. A. Appropriate use of Imlifidase; B Safe use of Imlifidase. C Minimize cold ischemia time.
Alternative to Imlifidase
According to the data from NHSBT, there has been an increase of 10% in transplantation in these highly sensitized patients after the change in the Kidney offering scheme 2019 (KOS). It is well known that patients who are very highly sensitized, that is, >99.5% CRF comprise about 10% of the waiting list. Thus, even if the new KOS has increased the transplantation rate by 10%, which equates to 1% of the very highly sensitized cohort, the remaining 9% (i.e. 90% of this group) will benefit from Imlifidase. Stewart et al.34 AJT, 2016, showed that the rate of transplant in the group who have greater than >99.95% cRF is significantly less than those with lower cRF, despite the changes in their allocation policy. They also showed that there is a bolus effect whereby the rate increased initially but reduced later. Another study showed that the transplant rate for candidates with cPRA >99.9% is six times less than that for candidates with a cPRA 99.5–99.9% despite both groups receiving similar priority under the revised kidney allocation system.35 Additionally, about 45% of the very highly sensitized (>99.5% cRF) wait for over 7 years on the waiting list. Drugs like Imlifidase would be the only option to be able to transplant this cohort. The alternative for not having a transplant with Imlifidase is to wait on dialysis. Studies have irrevocably shown that the quality of life on dialysis is poor when compared to transplantation.36
Moreover, the highly sensitized group will be increasing constantly due to the use of expanded donor criteria and fast-track organs. As Metzger et al.37 pointed out in AJT 2003, the use of these organs would result in increasing sensitization as these grafts do not last as long as standard deceased donor grafts. Every new patient joining the national waiting list would further disadvantage the existing highly sensitized group. Thus, though the changes in the national allocation policy can improve the chances of a transplant in this highly sensitized group, the need for a drug like Imlifidase still remains very high to achieve reasonable equity in this group of patients.
Limitations
Equating the immunological risks for Imlifidase and PP is notional, but there are similarities that suggest this is justified. Firstly, both only provide transient effective antibody elimination (functional elimination with Imlifidase). Secondly, there is patient variation in the post-transplant DSA dynamics with each, including that characterized by an early rebound and spontaneous modulation. Finally, the rates of early rejection are similar. Unlike PP, Imlifidase also directly removes the Fc fragment from cell-bound IgG, including on B cells. This may suppress B cell activation in the early post-transplant phase with the possibility that using Imlifidase might give a lower immunological risk than PP. However, while patient selection is based on notional risks, outcomes will be dependent on the response to actual events, the management of rejection, etc. Again, the experience and evidence from PP-based AIT should be applicable to Imlifidase-based transplants.
The management of individual cases, including the response to a DSA rebound, treatment of rejection, and graft dysfunction, should be based on appropriate evidence collected from PP cases. However, it must be remembered that although it is speculated that treatment outcomes with Imlifidase are likely to be similar to traditional desensitization procedures like PP, there is no long-term data from Imlifidase-treated patients available currently.
The standard triple therapy immunosuppression is the same for patients treated with Imlifidase. However, using human monoclonal or polyclonal IgG antibodies drugs like Alemtuzumab, Basiliximab, Rituximab, or rabbit ATG during the early phase will be ineffective as the IgG antibodies in the drugs are cleaved by Imlifidase. It is therefore recommended to wait for at least 4 days post Imlifidase to give these drugs.20 Intravenous Immunoglobulin (IVIG) possibly contains neutralizing antibodies against Imlifidase and, therefore, may inactivate Imlifidase, especially if IVIG is given before Imlifidase treatment. However, it can be given after 12 hours if need be.38 Equine ATG and Eculizumab are not cleaved by Imlifidase and hence can be given at any time. Furthermore, anti-imlifidase antibodies can occur 1-2 weeks after treatment with Imlifidase and, therefore, may impact further treatment with Imlifidase.38
Lastly, the drug is very expensive and might be a limiting factor for use in India. The cost aspects of the drug should certainly be discussed and negotiated with the company.
Conclusion
So, do we need a magic wand, and is it Imlifidase? The answers are perhaps yes and no. Currently, deceased organ transplantation is a competitive process with allocation based on a balance between equity and utility. The balance does change with each allocation scheme in the UK, recently with progression towards equity. A lack of sufficient donors is certainly the biggest obstacle to surpass. Ideally, we need a donation rate that matches the rate of demand. However, that would not fully address the demand because what is actually needed is a matching supply of compatible donors in order to eliminate a waiting list. This, of course, will be unachievable, but a practical alternative is to exclude the requirement for compatibility (in the cases where this is not possible): antibody removal or inactivation. As such, Imlifidase is not a magic wand, but it is a real and proven option. The value of the drug in helping the 8-10% of the individuals on the waiting list get a life-line cannot be overlooked. The alternative of staying on dialysis with the associated complications and impact on the health economy cannot be discounted.
Conflicts of interest
There are no conflicts of interest.
References
- Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney transplantation: The challenge of human leukocyte antigen and its therapeutic strategies. J Immunol Res. 2018;2018:5986740.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplant Proc. 2012;44:222-5.
- [CrossRef] [PubMed] [Google Scholar]
- The human leukocyte antigen system … simplified. Glob J Transfus Med. 2017;2:77-88.
- [Google Scholar]
- Calculated PRA (CPRA): The new measure of sensitization for transplant candidates. Am J Transplant. 2010;10:26-9.
- [CrossRef] [PubMed] [Google Scholar]
- Highly successful and low-cost desensitization regime for sensitized living donor renal transplant recipients. Ren Fail. 2009;31:533-7.
- [CrossRef] [PubMed] [Google Scholar]
- Successful removal and prevention of resynthesis of anti-HLA antibody. Transplantation. 1984;37:254-5.
- [CrossRef] [PubMed] [Google Scholar]
- Human leukocyte antigen antibody-incompatible renal transplantation: Excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92:900-6.
- [CrossRef] [PubMed] [Google Scholar]
- Donor-specific antibodies in kidney transplant recipients. Clin J Am Soci Nephrol. 2017;13:182-92.
- [Google Scholar]
- HLA antibody incompatible renal transplantation: Long-term outcomes similar to deceased donor transplantation. Transplant Direct. 2021;7:e732.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS–a novel therapeutic opportunity. PLoS One. 2015;10:e0132011.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The bacterial enzyme IdeS cleaves the IgG-type of B cell receptor (BCR), abolishes BCR-mediated cell signaling, and inhibits memory B cell activation. J Immunol. 2015;195:5592-601.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc Natl Acad Sci U S A. 2009;106:17864-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am J Transplant. 2018;18:2752-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunoglobulin fragment F (ab’) 2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antiviral Res. 2020;182:104868.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377:442-53.
- [CrossRef] [PubMed] [Google Scholar]
- IdeS (Imlifidase): A novel agent that cleaves human IgG and permits successful kidney transplantation across high-strength donor-specific antibody. Ann Surg. 2018;268:488-96.
- [CrossRef] [PubMed] [Google Scholar]
- Imlifidase as a potential treatment for antibody-mediated rejection. Curr Transplant Rep. 2021;8:157-61.
- [Google Scholar]
- Dynamic behaviour of donor specific antibodies in the early period following HLA incompatible kidney transplantation. Transpl Int. 2022;35:10128.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Successful treatment of acute antibody-mediated rejection of liver allograft with Imlifidase: A case report. Transpl Rep. 2023;8:100145.
- [Google Scholar]
- Imlifidase desensitization in crossmatch-positive, highly-sensitized kidney transplant recipients: Results of an international phase 2 trial (Highdes) Transplantation. 2021;105:1808-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outcomes at 3 years post-transplant in imlifidase-desensitized kidney transplant patients. Am J Transplant. 2021;21:3907-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76-85.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of alloantibody production in sensitized renal allograft recipients. Am J Transplant. 2009;9:998-1005.
- [CrossRef] [PubMed] [Google Scholar]
- Overview | Imlifidase for desensitisation treatment before kidney transplant in people with chronic kidney disease | Guidance | NICE. https://www.nice.org.uk/guidance/ta809/resources/imlifidase-for-desensitisation-treatment-before-kidney-transplant-in-people-with-chronic-kidney-disease-pdf- 82613258287045
- Quantifying the risk of incompatible kidney transplantation: A multicenter study. American Journal of Transplant. 2014;14:1573-80.
- [Google Scholar]
- Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8:2684-94.
- [CrossRef] [PubMed] [Google Scholar]
- The UK national registry of ABO and HLA antibody incompatible renal transplantation: Pretransplant factors associated with outcome in 879 transplants. Transplant Direct. 2017;3:e181.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16:1834-47.
- [CrossRef] [PubMed] [Google Scholar]
- Identifying sensitized kidney candidates with markedly low access to deceased donor transplantation by granular CPRA and Blood type. OBM Transplant. 2021;5
- [CrossRef] [Google Scholar]
- Health-related quality of life compared between kidney transplantation and nocturnal hemodialysis. PLoS One. 2018;13:e0204405.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114-25.
- [CrossRef] [PubMed] [Google Scholar]
- Imlifidase for the treatment of anti-HLA antibody-mediated processes in kidney transplantation. Am J Transplant. 2022;22:691-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







