Translate this page into:
Immune-Mediated Glomerulonephritis as Type 2 Lepra Reaction Posttreatment of Lepromatous Leprosy: A Case Report
Corresponding author: Divya Kantak, Department of Medicine, The Grant Government Medical College and Sir J.J. Group of Hospitals, JJ Marg, Nagpada-Mumbai Central, Near Sandhurst Road and J J Police Station, Mumbai, Maharashtra, India. E-mail: divyakantak@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kantak D, Dsouza M. Immune-Mediated Glomerulonephritis as Type 2 Lepra Reaction Posttreatment of Lepromatous Leprosy: A Case Report. Indian J Nephrol. 2024;34:520-1. doi: 10.25259/ijn_536_23
Abstract
Leprosy, an infectious disease known for its debilitating effects on the skin and nerves, can trigger immunologic reactions affecting multiple organs. We present the case of a 57-year-old male who developed acute glomerulonephritis following leprosy treatment. Clinical examination revealed newly developed pitting edema in the legs, along with residual nerve thickening and skin changes. Laboratory findings showed elevated serum creatinine (3.2mg/dl) accompanied by low C3 and C4 levels. Urinalysis supported the diagnosis of glomerulonephritis. Renal biopsy demonstrated immune complex deposition on immunofluorescence, suggesting a diagnosis of leprosy-related post-treatment immune-mediated glomerulonephritis. Treatment with oral steroids led to complete resolution of the condition.
Keywords
Leprosy
Glomerulonephritis
Type 2 lepra reaction
Nephritic syndrome
Acute kidney injury
Introduction
Leprosy (also known as Hansen’s disease) is a chronic illness caused by Mycobacterium leprae complex (Mycobacterium leprae and Mycobacterium lepromatosis), with a predilection for Schwann cells in the peripheral nerves and skin. Systemic involvement can occur in multibacillary forms. Reactional states are immune-mediated systemic inflammatory exacerbations that may affect 30%–50% of all leprosy patients.1 Type 2 lepra reactions are Type III hypersensitivity reactions caused by immune complex deposition in organs and tissues. Renal involvement in Type 2 lepra reactions is uncommon, but may present as glomerulonephritis, interstitial nephritis, or secondary amyloidosis.
Case Report
A 57-year-old male presented with gradually progressive bilateral lower limb swelling, facial puffiness, breathlessness, and generalized weakness over a period of 10 days. He had a history of borderline lepromatous leprosy one and a half year ago or 18 months ago, and had completed a 1 year course of multibacillary leprosy treatment 4 months before the onset of his current symptoms. He had no history of hypertension, diabetes, or chronic renal disease.
On examination, his pulse was 85 bpm and blood pressure was 130/80 mmHg. There was bilateral symmetrical painless pitting edema up to the knees and visible jugular venous pulsation 2 cm above the sternum. He exhibited coarse facial features, bilateral madarosis, hypopigmented patches over the distal limbs and torso, hyperpigmented patches over the body, [Figure 1] and painless nerve thickening involving the bilateral ulnar, common peroneal, right superficial radial, and right supraorbital nerves. Ichthyosis of bilateral lower limbs was present.
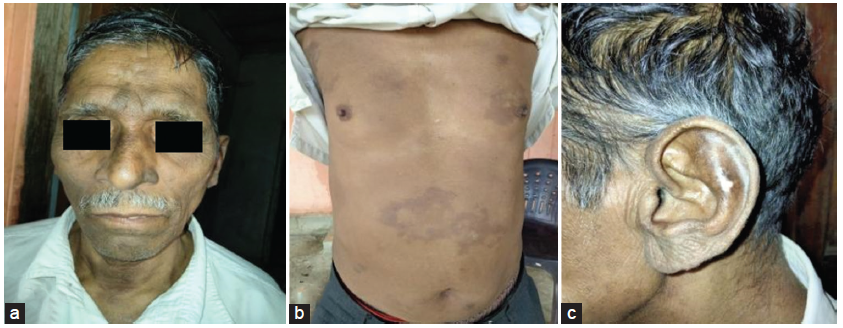
- Clinical presentation: (a) coarse facial features, madarosis, and hyperpigmented patches; (b) hypo- and hyperpigmented patches over the torso; and (c) patches over the earlobe.
Slit-skin smear samples were taken from areas of hypopigmentation from four sites, which revealed M. leprae with a bacteriological index of 3+ and a morphological index of 0, suggestive of adequately treated inactive leprosy.
Laboratory investigations showed hemoglobin: 11.2 g/dl, leukocyte count of 8300/mm3, platelet count of 178,000/mm3, serum creatinine: 3.2 mg/dl, estimated glomerular filtration rate (eGFR) of 22 ml/min, serum urea: 32 mg/dl, serum albumin: 3.8 g/dl, erythrocyte sedimentation rate (ESR): 60 mm/h, and C-reactive protein (CRP): 2.4 mg/dl. Urinalysis showed protein: 2+, red blood cells (RBC): 20–25/hpf, cast, crystal, bacteria: absent, and 24-h urine protein: 551 mg/day.
Electrocardiogram (ECG), chest radiograph, arterial blood gas, and 2D echocardiography showed no evidence of left ventricular or right ventricular dysfunction. Ultrasound (USG) abdomen and pelvis indicated normal kidney sizes, and computed tomography of the kidneys, ureters, and bladder (CT KUB) ruled out obstructive uropathy.
A provisional diagnosis of acute nephritic syndrome was established. Immunological workup showed the following: antinuclear antibody (ANA): 1:100 weakly positive speckled pattern, extractable nuclear antigens (ENA) panel- negative, C3 and C4: low, cytoplasmic anti-neutrophil cytoplasmic antibody (c-ANCA) and perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA): negative, RA factor, anti-cyclic citrullinated peptide (anti-CCP): negative.
Renal biopsy showed evidence of diffuse proliferative glomerulonephritis with endocapillary proliferation, mesangial hypertrophy, and increase in tuft size of glomeruli in the biopsy specimen, IgG1 (2+), C3 (3+), kappa and lambda (2+), granular mesangial and capillary wall deposits [Figure 2]. No bacilli were seen in the specimen.
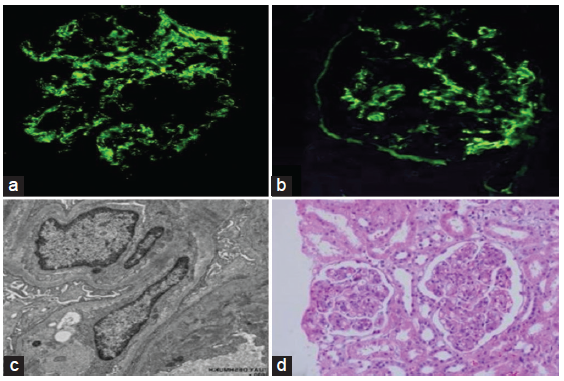
- Renal biopsy: (a) immunofluorescence showing granular mesangial and endocapillary C3 deposits; (b) immunofluorescence showing granular endocapillary and mesangial IgG deposits; (c) electron microscopy; and (d) light microscopy.
He was treated with loop diuretics for edema and started on oral prednisolone 60 mg (1 mg/kg) for 1 month, tapered over the next 6 weeks to a minimum dose of 5 mg. On follow-up, the patient’s proteinuria and hematuria resolved and serum creatinine normalized. Patient was adequately treated for leprosy as evidenced by a morphological index of 0 prompting no further treatment with multidrug therapy.
Discussion
Hansen’s bacilli antigens are released into circulation after the beginning of antibiotic therapy. They are recognized by host antibodies, leading to immune complexes, which can deposit in the glomerulus or occur in situ. Risk factors for renal involvement included lepra reaction, especially erythema nodosum leprosum, and advanced age.2 Not all glomerulonephritis in leprosy are associated with erythema nodosum, suggesting multifactorial influences in the development of leprosy nephropathy. In the Virchowian form, cellular immunity decreases and humoral immunity hyperactivates, making the patient susceptible to immune complex formation.1 In this case, immune complex-mediated glomerulonephritis occurred many months after completing multidrug treatment. A bacteriological index of 3+ with a morphological index of 0 suggests that the presence of killed bacilli may have induced an immune response, which is a rare occurrence. Anti-dapsone antibodies have been detected in the circulation of leprosy patients. Uremia due to chronic glomerulonephritis is a major cause of death in lepromatous leprosy, and proteinuria, microscopic hematuria, cell casts, and edema suggestive of glomerulonephritis are not unusual in reactional episodes.3 Leprosy has not usually been thought of as a cause of glomerulonephritis, and kidney parenchyma is not usually invaded in this disease.4
Conclusion
This case report highlights the importance of having a high degree of suspicion of renal involvement, which is not always rapidly progressive, even after effective treatment of leprosy. Renal involvement occurring in the absence of erythema nodosum is unusual. Prompt diagnosis and treatment with immunosuppressants prevent disease progression.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Leprosy nephropathy: A review of clinical and histopathological features. Rev Inst Med Trop Sao Paulo. 2015;57:15-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hansen’s disease with lepra reaction presenting with IgA dominant infection related glomerulonephritis. Indian J Pathol Microbiol. 2020;63:289-91.
- [CrossRef] [PubMed] [Google Scholar]
- Lepromatous leprosy presenting with polyarthritis, myositis, and immune-complex glomerulonephritis. Br Med J. 1975;3:619-21.
- [CrossRef] [PubMed] [Google Scholar]
- It is time to review concepts on renal involvement in leprosy: Pre- and post-treatment evaluation of 189 patients. Ren Fail. 2015;37:1171-4.
- [CrossRef] [PubMed] [Google Scholar]







