Translate this page into:
Immunoadsorption Column Reuse
Address for correspondence: Dr. Vaibhav Tiwari, Department of Nephrology, Sir Ganga Ram Hospital, Old Rajinder Nagar, New Delhi - 110 060, India. E-mail: drvt87@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Selective immunoadsorption (IA) is a technique to remove preformed Anti-ABO antibodies in ABO-incompatible renal transplants (ABOiRT). Since the cost of a single IA column is high and single use rarely achieves the target anti-ABO titers, its use is not widely spread. We studied the safety and efficacy of the reuse of IA columns in ABOiRT.
Methods:
Single-center, retrospective analysis of all patients who underwent ABOiRT with IA column reuse from January 2016 to July 2018. The column was reused after sterilization with ethylene oxide and flushed with normal saline before use. Target titers (IgG) were 1:4 preoperatively. Baseline IgG titers, plasma volume processed in each session, postoperative titer rebound were recorded. The primary outcome was IgG titer reduction after each use and adverse reaction during the IA column reuse. Patients were followed up until 1 year.
Results:
16 patients underwent ABOiRT using IA columns. Baseline IgG titer ranged from 1:32 to 1:512. Reuse of IA column was done 23 times and underwent 2nd reuse for 9 times. The average plasma volume treated was 22 L. Efficacy of the IA column in log titer reduction of anti-ABO titer was 4 logs after the first use, 3 logs after 1st reuse, and 1.5 logs after 2nd reuse. 12 (75%) patients successfully reached the target IgG titer of ≤1:4 solely with column reuse. One patient received a single session of plasma exchange before transplantation. Postoperatively, one patient received one session of plasma exchange due to a rebound in anti-ABO antibodies. No serious side effects were noted during the reuse.
Conclusion:
IA column reuse up to two times showed efficacy in the successful reduction of antibody titers. Column reuse was not associated with any significant side effects.
Keywords
ABO-incompatible Transplant
immunoadsorption column
kidney transplant
plasma exchange
reuse of IA columns
selective IA columns
Introduction
Transplantation is the treatment of choice for end-stage renal disease.[1] The disparity between demand and supply has blocked many chronic kidney disease (CKD) patients' chances to receive the treatment of choice for this chronic illness. Apart from a live related donor, cadaveric donation, paired kidney donation, and ABO-incompatible renal transplant (ABOiRT) have been other methods that can fill in the void. The ABOiRT, which was previously most common in Japan, has now been picking up in other countries as well.[2] One disadvantage of ABOiRT is the significant immunosuppression before the transplant to eliminate the anti-ABO antibodies. Splenectomy has completely been replaced by rituximab in most parts of the world. Plasma exchange (PLEX) is generally done to remove the already existing antibodies and is currently the most widely performed procedure for antibody removal pre-transplant. Since PLEX is a non-selective method, it also removes protective antibodies along with clotting factors leaving patient with increased risks of bleeding and infectious complication. Selective immunoadsorption (IA) technique provides a more specific approach to get rid of anti-ABO Antibodies without the undesirable side effects of PLEX.[3] It is generally recommended that the patient with high baseline titer should be treated with the IA column as this method is effective in reducing high titers in single use.[4] Nevertheless, as we have experienced in our center that single-use even with large plasma volume most of the time are not able to achieve the desired target titers. As the use of the IA column puts a considerable burden on a total expenditure of transplantation, reuse of the columns more than once is mostly performed. Studies assessing the adequacy of the reuse of the IA column are lacking. We have done a retrospective analysis of patients who underwent antibody removal by the IA column regarding its efficacy in reducing titer and safety of reuse of the IA columns.
Methods
We retrospectively analyzed all the patients who underwent ABOiRT using the IA column from January 2016 to July 2018.
Protocol for IA
Patients underwent the IA column with a target of approximately 8 plasma volumes in a single session. Maximum three sessions were scheduled with a single IA column, including 1st use, and two reuses every alternate day if required. If even after the 3rd session, titer was not achieved, the patient underwent PLEX sessions until the desired titer is reached. For decision-making, only the IgG antibody titer was used. Target titers were 1:4 preoperatively. Selective anti-ABO antibody removal was performed by Glycosorb®-ABO, which is a low molecular weight carbohydrate column containing A or B blood group antigens linked to a sepharose matrix (Glycorex Transplantation, Lund, Sweden). Clinical and laboratory investigations were noted, including anti-A or anti-B (IgG and IgM) titers, according to the case. IA procedure was started between 10 AM to 12 PM. IA column was connected to plasma filter, and blood flow was adjusted to provide a plasma flow at 40–50 mL/min to the IA column. The approximate duration of the session was 8–12 h. Titer was sent the next morning at 8 AM approximately 12 h after the procedure. IA was performed every alternate day. If titer was <1:8, transplantation was proceeded on the same day [Figure 1].

- Flowchart showing Immunoadsorption protocol. PLEX: plasma exchange
Postoperatively ABO antibody (IgG and IgM) titers were measured daily at 8 AM. Rescue PLEX was planned if titer (IgG) rises >1:16. If the rise of titer persist or rise in serum creatinine (>0.3 mg/dL) in 48 h occurs with titers ≥1:16, a kidney biopsy was planned. After 2 weeks, the anti-ABO titer was done weekly or whenever there was an unexplained rise in creatinine till 6 weeks.
Reuse of column
After each use, the column was flushed with 1 L of normal saline. Subsequently, it was sterilized with ethylene oxide at 55°C and the entire procedure was completed in 10 h. Later, the column was labeled and kept in the dark place at 2–8°C for at least 12 h before the reuse. Before the next use, the label was checked, and visual inspection was done to look for any break, discoloration, or visual clot. Before connecting to the circuit, the column was flushed with 1 L of normal saline. Anti-ABO antibody (IgG) antibody titer against donor ABO blood group antigen was measured in the recipient using column agglutination technology (also known as gel method) using ORTHO VISION® Analyzer.
Desensitization and immunosuppression protocol
Recipients received rituximab 375 mg/m2 15 days before the prospective date. Tacrolimus (0.05 mg/kg) and mycophenolate mofetil (1 g/day) were started at 14 days. On day 2, the dose of tacrolimus was increased to 0.1 mg/kg and mycophenolate mofetil to 2 g/day. Anti-thymocyte globulin (1 mg/kg) was given on day 0 to day 2. Injection methylprednisolone (500 mg/day) was given from day 0 to day 2.
Antibody (IgG) titer reduction with the IA column after each reuse, adverse reaction during the reuse of the IA column, post-transplant antibody (IgG) rebound and one-year patient and graft outcome were analysed.
Statistics
Data were analyzed using descriptive statistics. When the data were normally distributed, the mean and standard deviation were calculated. When the data were not normally distributed, the median and range were determined. Between-group comparison of numeric parametric data was done by unpaired t-test and P ≤ 0.05 was considered statistically significant.
Results
A total of 105 patients underwent an ABOiRT between January 2016 and July 2018. Sixteen patients underwent a selective IA column before transplantation. All patients were first-time prospective recipients. The mean age of the patient was 43 years. 90% of the patients were male recipients. The mean donor age was 46 years. 86% of the donors were female. O blood group was the most common among the recipients. Spousal donation especially wife to the husband was the most frequent relationship among donor-recipient groups. Among the blood group constellations were as follows: A to O (11 patients), B to O (one patient), A to B (one patient) and B to A (three patients).
Immunoadsorption sessions
We divided all the patients into four groups as per baseline titers [Table 1]. The baseline titers (IgG) ranged from 1:64 to 1:256. The median step titer reduction during the first use was 4 logs. With the first reuse reduction reduced to 3 logs (P = 0.02). With the second reuse, the efficiency further reduced to 1.5 logs (P = 0.006) when compared to the 1st use. We were able to achieve the target titer level (IgG), that is, ≤1:4 in 12 of the16 patients. 4 patients who did not achieve the target titer level received 1 or more sessions of PLEX. One of the four patients (patient 14) after one session of PLEX achieved 1:4 titer and, hence, proceeded to transplantation. Patient 8 underwent four additional PLEX sessions; his titers did not reduce from 1:32. Patient 9 underwent five sessions of PLEX, his titers reduced to 1:16 after 5th session, but it again rose to 1:64 the next day. Patient 7 underwent five sessions of PLEX, his titers did not reduce from 1:64. The mean plasma volume processed in each session was 22 L. The mean duration of each session was 10 ± 2.1 h. During the reuse, three patients complained of mild side effects like chills and rigors, but no serious adverse events were noted in any patients during reuse.
| (a): Anti-ABO Antibody Baseline titer 1:512 | ||||||
|---|---|---|---|---|---|---|
| Group I | Patient 3 | Patient 5 | Patient 7 | Patient 10 | ||
| 1st session | pre IA | 1:512 | 1:512 | 1:512 | 1:512 | |
| post IA | 1:16 | 1:16 | 1:256 | 1:32 | ||
| 1st reuse | Pre IA | 1:32 | 1:32 | 1:128 | 1:128 | |
| post IA | 1:04 | 1:04 | 1:64 | 1:08 | ||
| 2nd reuse | pre IA | Tx | Tx | 1:64 | 1:32 | |
| post IA | - | - | 1:64 | 1:04 | ||
| (b): Anti-ABO antibody baseline titer 1:256 | ||||||
| Group II | Patient 1 | Patient 2 | Patient 4 | |||
| 1st session | pre IA | 1:256 | 1:256 | 1:256 | ||
| post IA | 1:08 | 1:16 | 1:04 | |||
| 1st reuse | Pre IA | 1:32 | 1:64 | Tx | ||
| post IA | 1:08 | 1:08 | - | |||
| 2nd reuse | pre IA | 1:16 | 1:08 | - | ||
| post IA | 1:04 | 1:04 | - | |||
| (c): Anti-ABO antibody baseline titer 1:128 | ||||||
| Group III | Patient 9 | Patient 11 | Patient 12 | Patient 13 | Patient 14 | |
| 1st session | pre IA | 1:128 | 1:128 | 1:128 | 1:128 | 1:128 |
| post IA | 1:32 | 1:04 | 1:16 | 1:04 | 1:08 | |
| 1st reuse | Pre IA | 1:32 | 1:64 | 1:64 | 1:32 | 1:32 |
| post IA | 1:32 | 1:04 | 1:08 | 1:04 | 1:08 | |
| 2nd reuse | pre IA | 1:64 | 1:16 | 1:16 | Tx | 1:32 |
| post IA | 1:32 | 1:04 | 1:04 | - | 1:16 | |
| (d): Anti-ABO antibody baseline titer 1:64 | ||||||
| Group IV | Patient 6 | Patient 8 | Patient 15 | Patient 16 | ||
| 1st session | pre IA | 1:64 | 1:64 | 1:64 | 1:64 | |
| post IA | 1:04 | 1:64 | 1:08 | 1:08 | ||
| 1st reuse | Pre IA | Tx | 1:64 | 1:16 | 1:16 | |
| post IA | - | 1:32 | 1:04 | 1:04 | ||
| 2nd reuse | pre IA | - | 1:64 | Tx | - | |
| post IA | - | 1:32 | - | - | ||
Postoperative course
Postoperatively all patients had titers ≤1:8 on day 1 [Figure 2]. Patients 3 and 13 had titers gone up to 1:16 on days 7 and 9, respectively. However, the next day, both patients had their titers declined to 1:8 spontaneously. Patient 7 had titer went up to 1:32 on day 10; as per protocol, he was given 1 session of rescue PLEX. His titer showed a declining trend from the next day, with no rise in Creatinine. His titers had remained stable in the following week. A follow-up of 13 patients showed as trends of creatinine over 12 months in Figure 3. No patient was lost to follow up. At the end of 1 year, graft and patient survival were both 100%. Mean creatinine at the end of 1 year was 1.72 ± 0.69 mg/dL. Mean eGFR (CKD-EPI) was 47.92 mL/min/1.73 m2. Five of 13 patients had creatinine more than 1.5 mg/dL at the end of 12 months. Two patients (patients 3 and 12) were diagnosed with BK virus nephropathy on kidney biopsy at 26 and 30 weeks, respectively. At last follow-up, both the patient had rising serum creatinine despite the reduction of immunosuppressive therapy and IVIG. 1 patient (patient 13) had DSA positive antibody-mediated rejection at 6 weeks which was successfully treated with five sessions PLEX and IVIg.
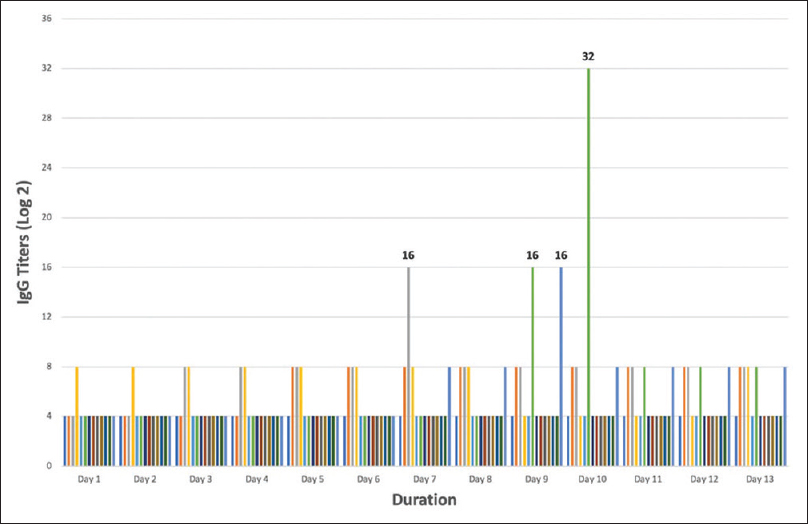
- Anti-ABO IgG antibody titer trend until the first 2 weeks after transplantation. Titers were monitored daily at 8 AM. Each bar represents a patient
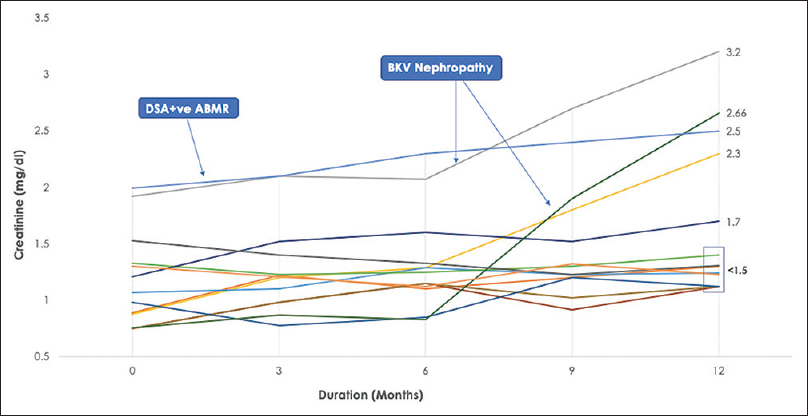
- S.Creatinine trend over 12 months. One patient diagnosed with DSA +ve ABMR in 2nd-month post-transplantation. Two patients diagnosed with BKV Nephropathy at 6 and 9 months. Box represents 61% patients with stable eGFR (57 ± 11.23 mL/min/1.732 m2) at 12 months. DSA+ve ABMR: donor-specific antibody-positive antibody-mediated rejection. BKV: BK virus
Discussion
This is the first study to report the safety and efficacy of the IA column reuse in ABOiRT from a developing nation. IA column use is still in the infancy stage in most parts of the world, including third world countries. Countries like the United States have still not adopted the IA column for its ABOiRT programs. So, the experience and knowledge regarding its use mainly come from European countries. A large meta-analysis that compared the IA column and plasmapheresis has shown better overall patient and graft survival when compared to plasmapheresis.[5].
Semi selective or non-selective IA columns (Immunosorba, Therasorba, Globaffin) in ABOiRT patients have been used successfully.[678] Their main advantage is the ease of reusability and cost-effectiveness.[9] The main drawback with these columns is nonselective nature hence the unpredictable nature of antibody reduction, especially when targeting anti-A/B antibodies.[10] Few small studies have compared the efficacy of selective versus non-selective IA columns.[711] Although non-selective IA columns are capable of efficiently removing blood group antibodies, they require a greater number of sessions and are more time-consuming. The reusability of selective IA columns can overcome this drawback along with providing a more cost-effective solution than the present single-use columns.
Previously, ABOiRT were performed by pre-operative plasmapheresis followed by splenectomy. In 2005, Tyden et al. introduced a protocol involving rituximab and selective IA columns session.[12] This was later referred to as the Stockholm protocol, which was subsequently adopted by many European countries.[13] Long term outcome of ABO-incompatible using selective IA columns has shown favorable outcomes.[1415] In a study by Wilpert et al. they compared the outcome of ABOiRT who used selective IA columns with ABO compatible transplant patients. They followed up the patients for more than 3 years.[14] Although the incidence of surgical complications like lymphocele was higher with ABOiRT, graft and patient survival, rejection, and infection rates were comparable in both the group. Tyden et al. compared 60 ABOiRT patients who underwent a selective IA column with 274 ABO compatible transplant patients with a follow-up of 5 years. Patient and graft survival were more than 95% in both groups.[15].
In this study, we evaluated the safety and efficacy of IA column reuse in ABOiRT. As per the company, the reuse of the column is not recommended as it may give rise to unwanted side effects and removal of non-selective antibodies. Since the price of one column is approximately 1/3rd price of total transplant expenditure, and single-use rarely achieves the desired target titers when baseline titers are high, reuse of column to see the efficacy and adverse effect profile was necessary.
In the present study, 23 times column was reused with 10 times as double reuse. We did not find any significant side effects which would have precluded the session. We found that Baseline titers do not entirely predict the successful outcome as patients from each subgroup [Table 1] except group II failed to reach the target titer. One of four patients did achieve the target titer with an additional single session of PLEX. Loss of efficacy of column on using multiple sessions could be cited as one the reason for failure, especially in 1st and 2nd reuse. This could be further complicated by the fact that patients with high titers have more antibodies which could saturate the columns during the first session only. There are two arguments against these. First, none of the patients in 1:256 baseline titers had a failure to achieve target titers, and second, all the patients who failed titer reduction by column were given a trial of plasmapheresis, hence reuse per se cannot be considered as a cause of failure to achieve target titers. Only one of four patients achieved a reduction from 1:16 to 1:4. This does not seem to be related to reuse and is observed after PLEX as well. Why some patients do not respond to the antibody removal technique is unknown. One possible explanation is that by removing the antibodies, the negative feedback on antibody-producing cells may be removed, leading to more proliferation and more production of antibodies until the feedback is once again activated.[16].
We used large volume plasma treatment (8 volumes= 2.5*8=20 L) per se ssion. This was excess in what was performed by Lionel et al. in their study (15 L). Session length was extended to 8–10 h, and we hypothesized that this would further lead to an increase in shift of antibodies from extravascular to the intravascular compartment and translating into less rebound as was suggested by their study.[17] We did get fewer rebound in most of the patients than as would have been expected when there is a large fall in titers in a single session. The method we used for regeneration was simple flushing with normal saline rather than using Immunosorba preservation solution manufactured by company for their non-selective filter regeneration as was adopted by Marc et al. in their study.[18] Since the column is not made for regeneration, we hypothesized that flushing with normal saline would wash out the antibodies and other components of blood, which might get stuck in the column and may give rise to unwanted side effects in the subsequent sessions. The Normal saline flushing of the column was shown efficacious in a case reported by Jha et al.[19]. We found that subsequent titer reduction efficacy of column decreases by almost 50% but remains superior to the single session of plasmapheresis. Postoperatively, the titers showed no rebound in most patients except in one patient who required an additional single session of PLEX.
In the study by Marc et al., they had reused the columns for 394 times for 54 patients with 3 sessions per column, five sessions per patient before Transplant, and 1–4 columns per patient. In the present study, the column was reused for 23 times for 16 patients, 2.5 sessions per column per patient were required before transplantation. That shows that we were able to reach the target titers in almost 50% fewer sessions, and with that single IA column was successful in reaching the target titers in 3/4th of the total patients. This was achievable since the plasma volume processed in a single session in the Marc et al. was average 2, and we had used a large plasma volume of 8.
At the end of 1-year follow-up, all patients showed 100% graft and patient survival with 1 patient (7%) showing DSA positive ABMR and two patients (15%) showing BKV Nephropathy. In the study by Marc et al., they had a similar rate of patient and graft survival at 1 year. During the follow up in their study, 18% of patients had developed BK viremia, but none had BK virus nephropathy. Adnan et al. in their study, reported an incidence of BKV nephropathy in 17.7% of 62 ABOiRT patients.[20] In a study by Jha, 50 ABOiRT patients with a mean follow up of 31 months who underwent PLEX as desensitization protocol, had an infection rate of 22% and was an important cause of mortality.[21] The benefit of IA over PLEX in terms of a decrease in infection rate needs a larger trial and at the moment is uncertain.
Our study has a few limitations. It is a single-center small study with a limited number of patients for a short follow-up period. Protocol allograft biopsy was not done. Nevertheless, literature is scarce in this field, and this report can guide future researches. The strengths of the study include meticulous adherence to protocol and rigorous follow-up with no attrition over 1 year.
Conclusion
The reuse of IA columns in ABO-incompatible transplants is safe. The efficacy of column decreases after the first use, but still, it can be used to treat high antibody titers, therefore, saving the cost and saving the patients from exposure to non-selective antibody removal techniques like plasmapheresis. A large plasma volume processing is feasible in a single session without compromising its antibody removal efficacy thereby reducing the total number of procedures and hospital stay. Anti A/B antibody has a stable course in the post-transplantation period without showing any significant rebound. Graft and patient outcomes are excellent and comparable to the ABO compatible transplants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Comparative analysis of ABO-incompatible kidney transplantation with ABO-compatible transplantation: A single-center experience from Eastern India. Saudi J Kidney Dis Transpl. 2019;30:97-107.
- [Google Scholar]
- Baseline anti-blood group antibody titers and their response to desensitization and kidney transplantation. Indian J Nephrol. 2017;27:195-8.
- [Google Scholar]
- Preconditioning therapy in ABO-incompatible living kidney transplantation. Transplantation. 2016;100:933-42.
- [Google Scholar]
- Present techniques for antibody removal. Transplantation. 2007;84(Supplement):S27-9.
- [Google Scholar]
- ABO-incompatible kidney transplantation enabled by non-antigen-specific immunoadsorption. Transplantation. 2012;93:827-34.
- [Google Scholar]
- ABO-incompatible kidney transplantation using regenerative selective immunoglobulin adsorption. J Clin Apher. 2012;27:51-60.
- [Google Scholar]
- ABO antibody and complement depletion by immunoadsorption combined with membrane filtration-a randomized, controlled, cross-over trial. Nephrol Dial Transplant. 2014;29:706-14.
- [Google Scholar]
- Anti-A/B antibody depletion by semiselective versus ABO blood group-specific immunoadsorption. Nephrol Dial Transplant. 2012;27:2122-9.
- [Google Scholar]
- Antigen-specific versus non-antigen-specific immunoadsorption in ABO-incompatible renal transplantation. PLoS One. 2015;10:e0131465.
- [Google Scholar]
- ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145-8.
- [Google Scholar]
- The Stockholm experience with ABO-incompatible kidney transplantations without splenectomy. Xenotransplantation. 2006;13:105-7.
- [Google Scholar]
- Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25:3778-86.
- [Google Scholar]
- Implementation of a protocol for ABO-incompatible kidney transplantation- a three-center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153-5.
- [Google Scholar]
- Treatment of large plasma volumes using specific immunoadsorption to desensitize ABO-incompatible kidney-transplant candidates. J Nephropathol. 2016;5:90-7.
- [Google Scholar]
- The reuse of immunoadsorption columns in ABO-incompatible kidney transplantation is efficient. Transplantation. 2015;99:1030-5.
- [Google Scholar]
- Reusing immunoadsorption column-making the ABO incompatible renal transplant affordable. Indian J Nephrol. 2017;27:241-2.
- [Google Scholar]
- Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:1320-7.
- [Google Scholar]
- ABO-incompatible renal transplantation: The journey so far on a road less traveled. Indian J Transplant. 2018;12:177.
- [Google Scholar]







