Translate this page into:
Impact of Water Treatment on Anemia in Hemodialysis Patients
Address for correspondence: Dr. Reem M. El-Sharabasy, Internal Medicine and Nephrology Department, Faculty of Medicine, Ain Shams University, Abbassia, Cairo - 11566, Egypt. E-mail: reemmohsen@med.asu.edu.eg
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The microbiological quality of water in the dialysate used in hemodialysis has been suggested as a contributor to inflammation, and a link between dialysate purity, inflammation, and responsiveness to erythropoietin therapy has been suggested in many studies. The level of endotoxin might induce inflammation and resistance to erythropoietin therapy in dialysis patients. We aimed to compare the effect of using the central dialysis fluid delivery system (CDDS) versus the single-patient dialysis fluid delivery system (SPDDS) on anemia in prevalent hemodialysis patients.
Methods:
In a prospective cohort study, 100 adult prevalent hemodialysis patients with T-SAT ≥20% were divided into two equal groups: CDDS and SPDDS. Endotoxin in water sample and routine investigations (hemoglobin, serum Ca+, serum Po4−, PTH, and urea level) were done. CRP, erythropoietin resistivity index (ERI), and erythropoietin stimulating agents (ESAs) doses were assessed repeatedly to assess inflammatory and anemia states.
Results:
Endotoxin level in the dialysis fluid of the CDDS group was significantly lower compared to the SPDDS group (0.05 vs. 0.11 EU/ml, P = 0.001). CRP level decreased significantly after 3 months in the CDDS group (P < 0.001) compared to the SPDDS group (P = 0.54), with significant improvement in the hemoglobin level and ERI at 3 months in the CDDS group and decrease in ESA requirements.
Conclusion:
Improvement in dialysis liquid purity reduces inflammatory markers in prevalent hemodialysis patients, improves ERI, and decreases ESA requirements in renal anemia.
Keywords
Anemia
CDDS
endotoxin
hemodialysis
Introduction
Prevalent hemodialysis patients undergoing hemodialysis (HD) three times/week, 4 h per session are exposed to a high water volume of 360–576 liters per week depending on their dialysate blood flow (500–800 mL/min). The quality of dialysis water is responsible for inflammation, which is a potent trigger of atherosclerosis and a pathogenic factor in anemia, increasing the mortality and morbidity in these patients. Therefore, this mandates the achievement of the highest level of purity of water coming into close contact with a patient’s blood.[1,2]
Currently, the available dialysis fluid delivery systems include the single-patient dialysis fluid delivery system (SPDDS) (individual dialysis fluid delivery system) and the central concentrates delivery systems (CCDS), in addition to the central dialysis fluid delivery system (CDDS). In SPDDS, dialysis fluid supply equipment is contained in the patient monitor. Nowadays, it is used widely and is considered a dialysis treatment global standard. Apart from the advantage of the relatively free location, SPDDS allows for the dialysis fluid composition individualization to meet unique patient needs.[3]
CDDS is especially useful in multi-patient treatment with in-center regular hemodialysis. It is a cost-effective, labor-saving, safe, and time-tested system with experience of almost 40 years. Microbial concerns have been cleared by a refined system design, tight RO membrane, placement of multiple endotoxin retentive filters (ETRF), and disinfecting the entire system on a daily basis.[4]
Endotoxemia is reported commonly in the dialysis population and has been linked to systemic inflammation. Endotoxins are much implicated in the pathogenesis of sepsis syndrome and are potent mediators of inflammation. Reported levels of endotoxemia in the dialysis population range from 0.209 to 2.31 endotoxin units/mL (EU/mL). Supposing an average 70-kg patient with approximately 3 L of circulating plasma volume, it is expected that as little as 0.12 EU/mL would be sufficient to trigger pyrogenic symptoms.[5]
The bacteriological qualities of the dialysis fluid water in CDDS were proven to be extremely high, having a strong impact on the patient outcome. The acceptable level of ET of dialysis fluid should be <0.1 EU/ml. The excellent water quality in CDDS might be one of the important factors of patient survival in chronic dialysis.[6]
Renal anemia, one of the most common complications in chronic kidney disease (CKD) that has deleterious effects on the patients’ quality of life, increases the risk of cardiovascular events and is associated directly and indirectly with various infections.[7]
The pathogenesis of anemia in CKD is usually multifactorial. The main features include the deficiency in erythropoietin (Epo) production and disordered iron balance. In addition to circulating uremia-associated inhibitors of Epo action, shortened survival of the red blood cell (RBC), iron losses, bleeding, and chronic inflammatory state can further aggravate the anemia.[8]
With such a proposed benefit of CDDS on dialysis water endotoxin levels and the deleterious effects of endotoxemia and its implication in the pathogenesis of anemia in this group of patients, we aimed to compare the effect of using CDDS and SDDS on anemia in prevalent hemodialysis patients.
Patients and Methodology
This is a prospective cohort study conducted in the hemodialysis units at Ain Shams University hospitals & Mansheiyat El-Bakry General Hospital, Cairo, Egypt between May and July 2019.
In total, 100 prevalent hemodialysis patients participated in this study. Adult patients with transferrin saturation of more than 20% were included. Patients with chronic infections and patients with other causes of anemia (malignancies, gastrointestinal bleeding) were excluded.
Study design and procedures
Patients in both groups were maintained on three sessions of hemodialysis of 4 h each with the use of high-flux dialysis membranes with surface area ≥1.8 m2.
Patients were assigned to two equal groups: group 1 had 50 patients on regular dialysis who were shifted to using CDDS shortly before the recruitment for the study at Ain-Shams University Hemodialysis unit, whereas group 2 had 50 patients on regular dialysis using SPDDS at Mansheiyat El Bakry General Hospital Hemodialysis unit.
Laboratory Investigations
Endotoxin assay
Endotoxin was measured in water samples from both dialysis fluid delivery systems using the Limulus amoebocyte lysate test (LAL test, by the Gel-clot technique to measure the degree of water purity in both systems) in addition to the routine investigations (serum Ca+, serum Po4-, PTH, and urea levels).
As a simple surrogate marker for the inflammatory status of the patients, C-reactive protein (CRP) was measured at baseline and on monthly basis throughout the study period (3 months) by using the latex serology test (Avitex CRP).
Anemia status in the participating patients was assessed by measuring the hemoglobin level on a monthly basis, together with EPO requirements that were assessed using EPO resistance index (ERI) (ERI = (EPO/wt)/Hgb),[9] calculated as average erythropoietin (EPO) dose per week per kg body weight (wt) per average hemoglobin (Hgb) to assess the response to erythropoietin therapy as a reflection of improvement of the patients’ inflammatory status. Satisfactory response was measured as an increase of hemoglobin at least 1.0 g/dL per month.
Ethical considerations
This study was performed in accordance with the ethical standards of Ain Shams University and with the 1964 Helsinki declaration and its later amendments of similar ethical standards. Informed consent was obtained from all patients participating in the study.
Statistical methods
IBM SPSS statistics (Statistical Package for Social Sciences) 2013, version 22.0, IBM Corp., USA, was used for data analysis. Descriptive statistics were done for continuous data as minimum and maximum of the range and mean ± standard deviation (SD). whereas for categorical data, it was done as number and percentage.
Continuous variables were tested using K–S test for normality testing, independent t test for two independent groups, and paired t test for two dependent groups. In categorical data, analyses were done using Chi-square test for differences between proportions. P < 0.050 was considered significant.
Results
No significant difference was noted between patients in both groups as regard age and sex. In the CDDS group, patients were maintained on dialysis for 3.4 ± 1.1 years, whereas SPDDS patients were dialyzing for 3.2 ± 0.9 years [Table 1].
| Measure | CDDS (n=50) | SPDDS (n=50) | P |
|---|---|---|---|
| Endotoxin level (EU/ml) | 0.05 | 0.11 | 0.001 |
| Age (years) | |||
| Mean±SD | 45.3±6.7 | 46.4±9.0 | 0.474 |
| Duration (years) | |||
| Mean±SD | 3.4±1.1 | 3.2±0.9 | 0.208 |
| Dry Weight (kg) | |||
| Mean±SD | 78.9±9.8 | 79.4±9.4 | 0.828 |
| Sex (n, %) | |||
| Male | 26 (52.0%) | 29 (58.0%) | 0.546 |
| Hemoglobin (gm/dL) | |||
| Mean±SD | 10.6±1.4 | 10.3±2.0 | 0.525 |
| Ca++ (mg/dL) | |||
| Mean±SD | 8.8±1.1 | 9.0±0.7 | 0.379 |
| PO4 (mg/dL) | |||
| Mean±SD | 4.2±1.7 | 4.6±2.0 | 0.230 |
| PTH (ng/L) | |||
| Mean±SD | 537.2±131.7 | 490.7±126.9 | 0.162 |
| Epo dose (IU) | |||
| Baseline | 7897.44±3611.35 | 9555.56±3484.20 | 0.047 |
| T-SAT (%) | |||
| Baseline | 37.49±16.25 | 35.51±15.92 | 0.259 |
| Ferritin (ug/L) | |||
| Baseline | 1119.61±852.40 | 1136.31±862.23 | 0.209 |
| URR | |||
| Baseline | 68.6±16.4 | 67.2±8.2 | 0.614 |
Ca: Calcium, PO4: Phosphorus, PTH: Parathyroid hormone, Epo: Erythropoietin, T-SAT: Transferrin Saturation, URR: Urea Reduction Ratio
Endotoxin level in the dialysis fluid of the CDDS group was significantly lower compared to the SPDDS group (0.05 vs. 0.11 EU/ml, P = 0.001).
Use of UPD yielded from CDDS dialysate improved the inflammatory status of this group of patients as reflected by a significant drop in the CRP level over 3-month follow-up (P < 0.001 for 1st, 2nd, and 3rd months) compared to the baseline in the study group, unlike the SPDDS group where CRP level exhibited no significant change through the 3-month follow-up period compared to baseline (P = 0.55, 0.197, and 0.54 for 1st, 2nd, 3rd months, respectively), with a significant difference between both groups (P < 0.001for 1st, 2nd, and 3rd months). [Figure 1]
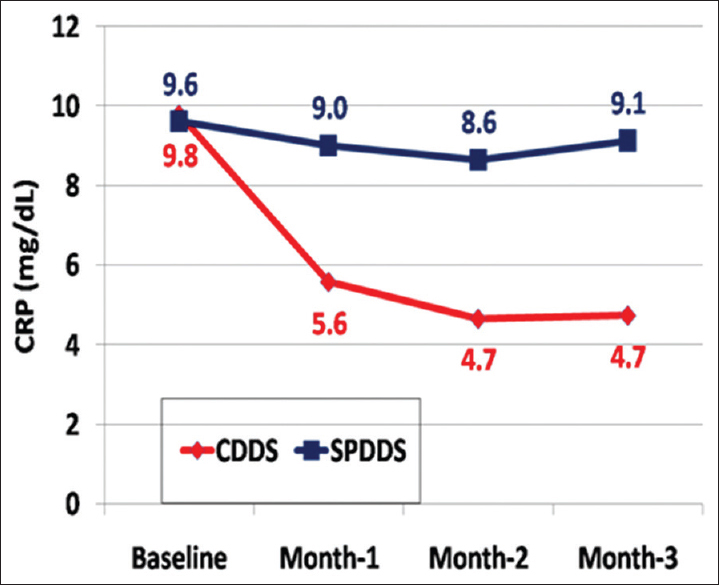
- Comparison of CRP level in both groups during the study period
The CDDS group also showed a significant sustained improvement in hemoglobin level (P = 0.008 for 1st month, <0.001 for 2nd and 3rd months) and ERI (P = 0.002 for 1st month, <0.001 for 2nd and 3rd months) over the 3-month follow-up compared to baseline, together with a decrease in their ESA requirements as reflected by decreased erythropoietin stimulating agents’ doses at 3 months (5217± 2539.80 Vs 10272.73 ± 2781.70 for CDDS & SPDDS respectively, P-value: 0.001) compared to baseline [Table 1], needed to maintain the same hemoglobin level.
Conversely, in the SPDDS group, there was a significant increase in ERI through the 3-month follow-up period (P = 0.039, 0.025, and 0.047 for 1st, 2nd, and 3rd months) associated with failure of improvement in Hb level all through the study period (P = 0.70, 0.14, and 0.149 for 1st, 2nd, and 3rd months) compared to baseline, with a significant difference between both groups (P = <0.001 for 1st, 2nd, and 3rd months for both hemoglobin and ERI) [Figures 2 and 3].
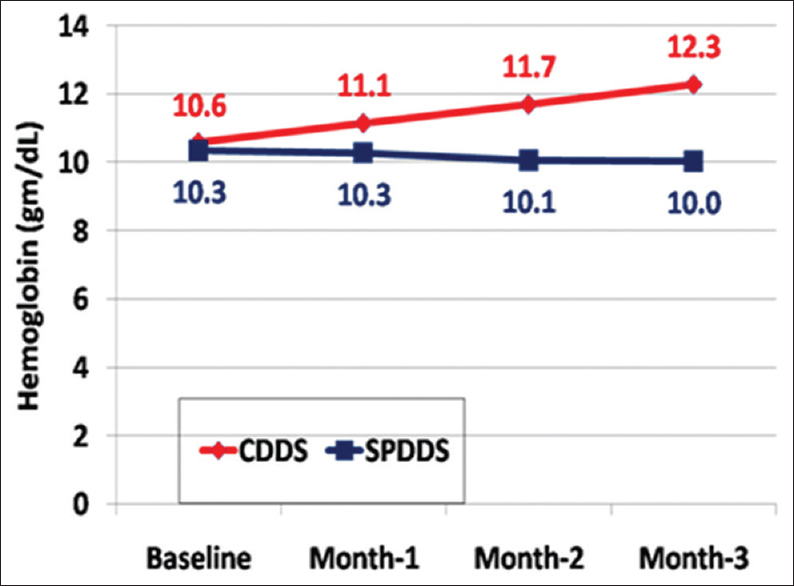
- Comparison of hemoglobin level in both groups during the study period
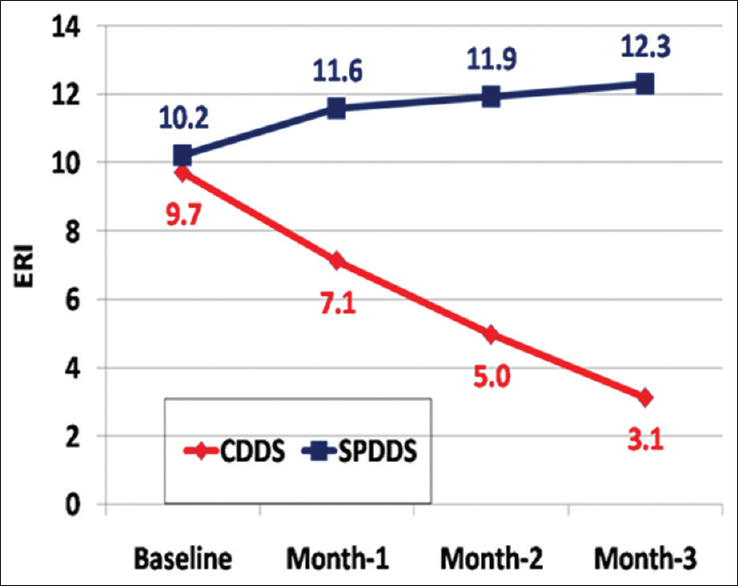
- Comparison of ERI level in both groups during the study period
Moreover, no significant correlation was detected between CRP as a representative of the inflammatory state and neither hemoglobin level nor ERI at baseline (P = 0.46 and 0.29, respectively) and after 3 months of follow-up (P= 0.32 and 0.51, respectively) in the CDDS group of patients [Table 2], unlike SPDDS group, there was a negative correlation between CRP and hemoglobin level (P = 0.019) after 2 months, together with a positive correlation between CRP and ERI (P = 0.018) after 2 months, further confirming that the worsened inflammatory state in these patients is associated with the poor response to EPO and low hemoglobin level [Table 3].
| Study group (CDDS) | CRP baseline | CRP after 3 months | ||
|---|---|---|---|---|
| r | P | r | P | |
| HB | ||||
| Baseline | −0.107 | 0.461 | ||
| 3 months | −0.142 | 0.324 | ||
| ERI | ||||
| Baseline | 0.153 | 0.290 | ||
| 3 months | 0.095 | 0.513 | ||
CDDS: Central dialysis delivery system, CRP: C-reactive protein, HB: Hemoglobin, ERI: Erythropoietin Resistance Index
| Control group (SPDDS) | CRP baseline | CRP after 2 months | ||
|---|---|---|---|---|
| r | P | r | P | |
| HB | ||||
| Baseline | −0.284 | 0.045 | ||
| 2 months | −0.33 | 0.019 | ||
| ERI | ||||
| Baseline | 0.282 | 0.047 | ||
| 2 months | 0.333 | 0.018 | ||
SPDDS: Single patient dialysis delivery system, CRP: C-reactive protein, HB: Hemoglobin, ERI: Erythropoietin Resistance Index
Furthermore, there was a significant decrease in ferritin level in the CDDS group at 3 months compared to baseline (1119.61 ± 852.40 versus 851.88 ± 599.50 respectively, P-value: 0.056), which might further indicate the aforementioned improvement in the inflammatory status in this group, together with a slight (although statistically insignificant) improvement in the T-SAT was also noticed in the CDDS group at 3 months follow up (36.99 ± 16.41, P-value: 0.066). The use of CDDS did not affect the URR in our study, baseline data are listed in table 1.
Discussion
The microbiological quality of the water in the dialysate used in hemodialysis treatment has been suggested as a major contributor to the inflammatory status in this group of patients with the background idea that microbiologic contaminants in the dialysate and level of endotoxin might induce inflammation and resistance to ESA in dialysis patients.[10,11]
In the current study, endotoxin level in the dialysis fluid of the CDDS group was found to be significantly lower compared to the dialysis fluid of the SPDDS group, which was associated with significantly lower levels of CRP level at 3-month follow-up compared to baseline (P < 0.001) in the same group, unlike the SPDDS group, with a statistically significant difference between both groups [Figure 1].
Similarly, Praditpornsilpa et al. showed that chronic inflammation in patients undergoing high-flux HD is minimized in the case of lower endotoxin contamination. They stated that chronic inflammation in HD is strongly associated with water quality and can be assessed by measuring the concentrations of endotoxin in the dialysate.[11]
These results are also coping with the meta-analysis of Susantitaphong et al.,[12] who examined ultrapure dialysate (UPD) versus standard dialysate effects on markers of inflammation, and anemia parameters by the analysis of 23 study arms or cohorts (n = 2221) where UPD resulted in a significant decline in CRP (P < 0.001).
Our study showed that improved inflammatory status of this group of patients was associated with a significant, sustained improvement in their hemoglobin level [Figure 2], ERI, and a decrease in their ESA requirements over the 3 months of follow-up that was also absent in the SPDDS group [Figure 3 and Table 2].
The results of Maoujoud et al. also showed that the use of UPD by applying endotoxin filter on conventional dialysate circuit is associated with better control of anemia in a follow-up of chronic HD patients; moreover, the ERI was significantly lower after conversion to UPD (P = 0.01) at 1 year.[10]
Again, the aforementioned meta-analysis by Susantitaphong et al.[12] stated that UPD resulted in a significant increase in hemoglobin level (P = 0.022) with a decrease in the weekly erythropoietin dose (P < 0.001).
These results are in concordance with the results by Hung et al.,[13] which yielded a positive correlation between high sensitivity CRP, IL-6, and TNF-serum levels with the required epoetin dose and epoetin responsiveness index. The results of this analysis support a strong relationship between high CRP levels and epoetin hypo-responsiveness in HD patients.
Our findings are also in agreement with the data reported by Ledebo, who had evaluated the effect of UPD on anemia management and reported signs of improved erythropoietin response, manifested either by decreased EPO requirements to maintain a certain Hb or by an increase in Hb levels in response to the same EPO dose.[14]
Our prospective study showed no effect of improved dialysate purity on the efficacy of the hemodialysis sessions as reflected by the urea reduction ratio (URR). Similarly, Molina et al.[15] stated that dialysis with ultrapure dialysis fluid (by placing a hydrophilic nylon filter (capable of trapping particles bigger than 0.22 microns) post-osmosis and a polysulfone filter before the dialyzer (Diasafe, Fresenius Medical Care®)) showed no significant differences as compared to baseline for Kt/V at any of the cut-off points.
However, our results conflict with Molina et al. where they showed an insignificant change in serum ferritin level, unlike our results, where there was a significant decrease in serum ferritin level at 3-month follow-up.[15]
Conclusion
UPD produced by the CDDS was positively reflected in minimizing the inflammatory status of this group of patients, together with a significant sustained improvement in their hemoglobin level, ERI, and a decrease in their ESA requirements, compared to the SPDDS.
Study limitations
-
Small sample size.
-
Data about IV iron prescription were not included in the patients’ medications.
-
Differences were observed between the groups in EPO doses at baseline, which is mainly because EPO doses are given on an individual basis according to every patient’s needs.
-
Inflammatory status was assessed by CRP level only; other inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR) and IL-6 were not assessed. We aimed at keeping it as simple as possible to make it easy to follow up the inflammatory status in these patients with a simple and easy test.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ultrapure water in haemodialysis: A step towards better quality in Lebanon. East Mediterr Health J. 2019;25:134-41.
- [Google Scholar]
- Erratum to: Ultrapure dialysis water obtained with additional ultrafilter may reduce inflammation in patients on hemodialysis. J Nephrol. 2017;30:883.
- [Google Scholar]
- The central dialysis fluid delivery system (CDDS): Is it specialty in Japan?Ren Replace Ther. . 2016;1:1. doi: 10.1186/s41100-016-0016-4
- [Google Scholar]
- Advances and advantages in recent central dialysis fluid delivery system. Blood Purif. 2009;27(Suppl 1):23-7.
- [Google Scholar]
- Is endotoxemia in stable hemodialysis patients an artefact?Limitations of the limulus amebocyte lysate assay and role of (1-->3)-beta-D glucan. PLoS One. 2016;11:e0164978.
- [Google Scholar]
- Bacteriological water quality in the central dialysis fluid delivery system from the survey of the Japanese Society for Dialysis Therapy. Blood Purif. 2009;27(Suppl 1):11-6.
- [Google Scholar]
- Levocarnitine injections decrease the need for erythropoiesis-stimulating agents in hemodialysis patients with renal anemia. Cardiorenal Med. 2017;7:188-97.
- [Google Scholar]
- Investigational therapies for renal disease-induced anemia. Expert Opin Investig Drugs. 2016;25:901-16.
- [Google Scholar]
- The greatly misunderstood erythropoietin resistance index and the case for a new responsiveness measure. Hemodial Int. 2016;20:392-8.
- [Google Scholar]
- Clinical and economic benefits of dialysate quality on anemia control and erythropoietin responsiveness among chronic hemodialysis patients. J Prev Epidemiol. 2016;1:e15.
- [Google Scholar]
- Effects of different levels of endotoxin contamination on inflammatory cytokine production by peripheral blood mononuclear cells after high-flux hemodialysis. Blood Purif. 2011;32:112-6.
- [Google Scholar]
- Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: A meta-analysis. Nephrol Dial Transplant. 2013;28:438-46.
- [Google Scholar]
- Erythropoiesis-stimulating agents in chronic kidney disease: What have we learned in 25 years?J Formos Med Assoc. . 2014;113:3-10.
- [Google Scholar]
- Ultrapure dialysis fluid--how pure is it and do we need it? Nephrol Dial Transplant. 2007;22:20-3.
- [Google Scholar]
- [Importance of ultrapure dialysis liquid in response to the treatment of renal anaemia with darbepoetin in patients receiving haemodialysis. Nefrologia. 2007;27:196-201.
- [Google Scholar]







