Translate this page into:
Influence of CYP3A5 and ABCB1 Polymorphism on Tacrolimus Drug Dosing in South Indian Renal Allograft Recipients
Address for correspondence: Dr. Manokaran Sellappan, Department of Nephrology, Govt. Stanley Hospital, Old Jail Road, Chennai - 600 001, Tamil Nadu, India. E-mail: manonephro@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Tacrolimus blood levels are influenced by polymorphisms involving Cytochrome 3A subfamily (CYP3A5) and P-Glycoprotein (ABCB-1) genes. However, their role in transplant outcomes was less studied in South Indian population. We studied the prevalence and impact of these polymorphisms in renal transplant recipients from South India.
Methods:
An analysis of CYP3A5, ABCB1 genotype done in 101 renal transplant recipients by polymerase chain reaction was correlated with blood tacrolimus trough levels (CLIA method), weight, concentration/dose (L/D) ratio, incidence of biopsy proven early acute rejections, and tacrolimus toxicity.
Results:
Prevalence of CYP3A5*1/*1, *1/*3 and *3/*3 and ABCB1 (3435C>T) TT, CT, CC genotypes were 12 (11.9%), 48 (47.5%), 41 (40.6%) and 16 (15.8%), 45 (44.6%), 40 (39.6%), respectively. Mean tacrolimus level, median concentration/dose (L/D) ratio were significantly lower in homozygous (CYP3A5*1/*1-6.01 ng/mL; 48.99 ng/mL/mg/kg/day) and heterozygous expresser group (CYP3A5*1/*3-5.84 ng/mL; 68.93 ng/mL/mg/kg/day) when compared with nonexpresser group [CYP3A5*3/*3-7.46 ng/mL (P < 0.001);181.3 ng/mL/mg/kg/day (P < 0.05]. No significant differences observed between the ABCB1 genotypic groups. Incidence of early acute rejections (30% vs. 9.76%; P 0.016) and tacrolimus-related toxicity (14.6% vs. 5%; P 0.039) were significantly higher in CYP3A5 expressers and nonexpressers, respectively. No correlation observed between the ABCB1 polymorphisms between rejection episodes or tacrolimus renal toxicity. Among 101 patients, 40.6% were non-expressers (poor metabolizers) (*3/*3).
Conclusions:
CYP3A5 polymorphisms correlated with tacrolimus dose requirements and blood levels, incidence of early acute rejection, and tacrolimus nephrotoxicity. CYP3A5 polymorphism analysis prior to renal transplant will aid more precise early tacrolimus dose calculation to balance between rejection and toxicity.
Keywords
Polymorphisms
rejection
renal transplant
tacrolimus
tacrolimus toxicity
Introduction
Tacrolimus is the vital component of renal transplant immunosuppressive therapy. However, because of its narrow therapeutic range, trough level monitoring is important for the prevention of its toxicity as well as rejection. Tacrolimus is metabolized by the liver cytochrome P450 CYP3A5 enzyme family.[1] CYP3A5 is expressed in the liver and catalyses the metabolism of tacrolimus.[2] CYP3A5 is also expressed in the small intestine and is thought to participate in the oral clearance of tacrolimus. A polymorphism in intron 3 of the CYP3A5 gene was found to influence the expression of this enzyme; CYP3A5*3 allele (G at position 6986) produces a cryptic splice site and encodes an abnormal spliced mRNA with a premature stop codon, while CYP3A5*1 allele (A at position 6989) produces a normal mRNA, resulting in a high expression of this enzyme in the intestine and in the liver.[34] Previous studies showed that renal graft recipients homozygous for the wild-type allele CYP3A5*1 required higher tacrolimus doses to achieve the target trough concentrations compared with carriers of the variant allele *3.[5678] Individuals possessing at least one CYP3A5*1 allele (CYP3A5 expressers) achieved twofold lower tacrolimus concentrations/dose ratio compared with CYP3A5*3/*3 homozygous (CYP3A5 nonexpressers).[9] Polymorphisms in intron 3 of this gene affects the metabolic activity of this enzyme.
The oral bio availability is also determined by the P-glycoprotein, an efflux pump encoded by ATP Binding Cassette 1 (ABCB1) gene, expressed in the apical membranes of epithelial cells, such as liver, kidney, and intestine, and it contributes to the elimination of drugs into the bile and urine. P-glycoprotein acts in synergy with the CYP3A subfamily in limiting intestinal absorption of various drugs.[10] Genetic polymorphisms in exon26 (3435C>T) were correlated with cellular expression level of P-glycoprotein in relation with ABCB1 mRNA stability and/or the protein's timing of cotranslational folding.[11] The effect of this ABCB1 polymorphisms on tacrolimus oral bioavailability remains controversial.[8] The effect of CYP3A5 and ABCB1 single nucleotide polymorphisms (SNPs) on tacrolimus pharmacokinetics are not well studied in South Indian population.
The purpose of this study was to identify the prevalence of CYP3A5, ABCB1 polymorphisms in South Indian kidney transplant recipients and to determine the effect of CYP3A5, ABCB1 gene polymorphisms on serum tacrolimus concentration (L/D ratio), incidence of acute rejections and Calcineurin inhibitortoxicity.
Patients and Methods
In total, 101 renal allograft recipients consented to participate in this study. The study population was recruited from the renal transplant outpatient clinic of our institution, between April 2017 and September 2017. Only patients with tacrolimus-based immunosuppressive treatment with stable graft function of >6-month duration were eligible to participate in the study. Patients with ongoing rejections, infections, with drugs that interfere with the tacrolimus levels, and abnormal liver function tests were excluded from the study. Our immunosuppressive protocol consisted of initial daily dose of tacrolimus was 0.1 mg/kg, given twice daily. Then, the dose was adjusted to obtain a trough blood concentration between 8 and 10 ng/mL for the first 6 months, followed by between 5 and 8 ng/ml. All patient's serum tacrolimus were obtained from their records, which were taken at the sixth month of post-transplant period. All whole blood tacrolimus trough concentrations were measured by chemiluminescence immunoassay (CLIA) from a single laboratory. Tacrolimus daily dose and patient's body weight were obtained from records of all patients at the time of trough level estimation. Level/dose ratio (ng/mL/mg/kg/day) was calculated for all patients. Peripheral blood samples for genotypic analysis obtained from all patients, during their regular visit to nephrology OPD (2 mL of Ethylenediaminetetraacetic acid preservative added and transported at 4°C temperature for genotyping). Biopsy-proven early acute rejection episodes (BPAR) reported during the first month of post-transplantation and the incidence of biopsy-proven CNI toxicity (biopsy findings correlated with clinical aswell as the blood C0 levels) were obtained retrospectively from patient's medical files. BPAR was considered for at least Banff 1 in severity according to classification of Banff '05.[12] Study was approved by the ethics committee of our institution. Protocols followed according to the ethical guidelines of the 1975 Helsinki Declaration. All patients were explained in detail about the study in their vernacular language and written consent obtained from all patients. The patients who participated in this study did not incur any expenditure.
Genomic DNA extraction and allele-specific polymerase chain reaction with melting point analysis
Genomic DNA was extracted from 0.2mL of peripheral blood by silica column-based DNA extraction kit (Cat# 51104, QiaAmp Blood DNA kit, Qiagen, Germany) as per manufacturer's protocol. Genotyping for CYP3A5 (A6986G)(rs776746) and ABCB1 (C3435T) (rs1045642) polymorphisms were performed by allele-specific polymerase chain reaction (PCR) in combination with melting point analysis (ASPCR-MPA). Briefly, 50ng of genomic DNA was subjected to allele-specific PCR with following primer pairs (Eurofins, Bangalore):
CYP3A5
Forward primer: CACTTGATGATTTACCTGCCTTC
Reverse primer-wild-type allele specific: GGTCCAA ACAGGGAAGAGATAT
Reverse primer- mutant allele specific: GGTCCAA ACAGGGAAGAGATAC.
MDR1 (ABCB 1)
Forward primer: ACTATAGGCCAGAGAGGCTGC
Reverse primer wild-type allele specific: GTGGTGTCACAGGAAGAGCTT
Reverse primer mutant allele specific: GTGGTGTCACAGGAAGAGCTC.
As described earlier by Ashavaid et al.,[13] the CYP3A5 primers produce a 218-bp Amplicon, whereas MDR1 primers produced 134-bp Amplicon. The allele-specific real-time PCR amplification of samples were performed with Type-IT HRM kit (cat #206542, Qiagen) in a Qiagen Rotor Gene Q real time PCR system (Qiagen) under following conditions: after an initial denaturation at 94°C for 4 min, samples were subjected to 35 cycles of denaturation at 94°C for 30 s, primer annealing at 60°C for 30 s, and primer extension at 72°C for 30 s. Following the amplification cycles, the samples were subjected to melting point analysis with 0.1°C/s to confirm the specificity of amplification and distinguish the amplification curve from possible primer dimers or nonspecific amplifications.
Statistical analysis
Data are described as mean ± SD and median ± 2SD for all quantitative estimates. To estimate the effect of CYP3A5 and ABCB1 genotypes on tacrolimus daily dose, we used one-way analysis of variance (ANOVA) test. For concentration/dose ratio of tacrolimus, we used Kruskal-Wallis test. Tacrolimus trough blood levels, L/D ratio and rejection episodes between two groups (expresser vs.nonexpresser) were compared by means of independent t-test. Chi-square test was used to test the risk of acute rejection and CNI toxicity between the three genotype groups. Fisher's exact test was used to test the risk of CNI nephrotoxicity in different groups. Statistical analysis was done using R- software version 3.4.2 (Stanford). For all the tests, P value < 0.05 was considered to be statistically significant.
Results
In total, 101 renal graft recipients with tacrolimus-based immunosuppressive treatment were included in the study. There were 85 (84.2%) men and 16 (15.8%) women. The mean age was 32 (27–38.5) years and body weight was 60.4 ± 13 kg. All patients were of South Indian origin. Most common Etiology for ESRD was unknown (73.3%); glomerular diseases accounted for 21% of ESRD (IgAN-7.9%, FSGS-5%). 70.3% were living related transplants and deceased donor graft recipients accounted for 29.7%. The median post-transplant duration was 32 months (16.5-53). Mean serum creatinine was 1.36 ± 0.3 mg/dLmg/dl. About 34.7% patients received induction therapy (ATG- 27, Basiliximab- 8). There was no significant differences noted in terms of age, sex, donor type (deceased or live), and induction agent usage between the CYP3 groups. However, we were not able to analyze the HLA match in the deceased donor and spousal donor transplants. All patients were on tacrolimus-based immunosuppression in combination with steroids and mycophenolate mofetil (n = 99), or steroids and azathioprine (n = 02). Twenty-two (21.8%) patients had early BPARs within the first month of transplantation. Overall, 8.9% (9 patients) had biopsy evidence of tacrolimus-related renal toxicity (within first year of transplantation). 5 patients in living donor group and 4 patients in deceased donor group had delayed graft function (7.1% and 13.34%). Mean donor age in living and deceased donor group was 51.6 years and 43 years (p 0.01) respectively. 18.8% patients had infection episodes and required admission in the first post-transplant year and NODAT was present in 19 (18.8%) patients.
The CYP3A5*1/*1,*1/*3, and *3/*3 genotypes were detected in 12 (11.9%), 48 (47.5%), and 41 (40.6%) of the 101 graft recipients, respectively. The ABCB1 (3435C>T) TT, CT, and CC genotypes were in 16 (15.8%), 45 (44.6%), and 40 (39.6%), respectively.
Effect of CYP3A5 genetic polymorphism on tacrolimus daily dose and concentration/dose ratio
The daily tacrolimus dose requirement was much higher for CYP3A5 expresser (*1/*1 and *1/*3) groups when compared with the CYP3A5 non-expresser (*3/*3) group [Graph 1]. Mean tacrolimus level in the CYP3A5*1/*1, CYP3A5*1/*3, and CYP3A5*3/*3groups were 6.01 ng/mL (SD = 4.53 ng/mL), 5.84 ng/mL (SD = 1.78 ng/mL), and 7.46 ng/mL (SD = 2.99 ng/mL), respectively, with the P value of <0.05 [Graph 2]. The heterogeneous expresser group (*1/*3) had lower mean Tac level when compared with the homogenous expresser group (*1/*1), which may be because of lesser patients in homogenous group (12 vs. 48) and the difference is not statistically significant (P > 0.05). Tacrolimus level difference between expressers and nonexpressers were significant when compared by using one-way ANOVA test. CYP3A5*1/*1,*1/*3 groups had significantly lower median concentration/dose (L/D) ratio than the CYP3A5*3/*3 non-expresser group (48.99, 68.93, and 181.3 (ng/ml/mg/kg) with the P value of <0.001) when compared by Kruskal-Wallis test [Graph 3]. Incidence of early acute rejections and CNI toxicity were compared between expresser (CYP3A5 *1/*1, *1/*3) and non-expresser genotypic groups (CYP3A5*3/*3). Expressers had significantly higher rejection rates when compared with nonexpressers (30% vs. 9.76%; P < 0.05). Meanwhile, nonexpressers had higher incidence of biopsy-proven CNI toxicity when compared with expressers (5% vs. 14.6%; P < 0.05) [Table 1].
| Characteristics | CYP3A5*1/*1 | CYP3A5*1/*3 | CYP3A5*3/*3 | P |
|---|---|---|---|---|
| Number of patients | 12 | 48 | 41 | 0.12 |
| Recipient age in years (mean) | 34.16 | 32.2 | 32.58 | 0.081 |
| Donor age in years (mean) | 47.91 | 49.92 | 49.13 | 0.11 |
| Male:Female | 10:2 | 38:10 | 37:4 | 0.11 |
| Live donors | 58.33% | 70.83% | 73.17% | 0.071 |
| Mean creatinine (mg/dL) | 1.27 | 1.31 | 1.45 | 0.088 |
| Tacrolimus dose (mg/day) (mean±SD) | 6.54(±2.17) | 5.08(±1.85) | 2.66(±1.21) | <0.001 |
| Tacrolimus level (ng/mL)-(mean) | 6.01(±4.54) | 5.84(±1.78) | 7.46(±2.99) | 0.019 |
| L/D ratio (ng/mL/mg/kg/day) | 48.99 | 68.93 | 181.3 | <0.001 |
| Induction treatment | 50% | 35.42% | 29.27 | 0.986 |
| Rejections | 3 (25%) | 15 (31.3%) | 4 (9.76%) | 0.047 |
| 18 (30%) | 4 (9.76%) | 0.016 | ||
| CNI toxicity | 1 | 2 (4.2%) | 6 (14.6%) | 0.158 |
| 3 (5%) | 6 (14.6%) | 0.039 | ||
CYP3A5: Cytochrome P450 family 3 subfamily A member 5

- Comparing the tacrolimus dose requirements between the CYP3A5 groups

- Comparing the blood tacrolimus levels between the CYP3A5 groups
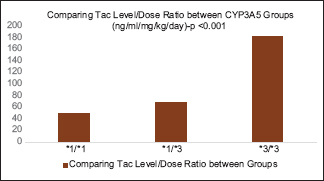
- Comparing the L/D ratio between the CYP3A5 Groups
Effect of ABCB1 (MDR1) genetic polymorphisms on tacrolimus daily dose and concentration/dose ratio
Our analysis showed that ABCB1 polymorphisms did not have significant effect on tacrolimus daily dose and concentration/dose ratio. Incidence of early acute rejections and CNI toxicity were not significantly different between ABCB1 (3435C>T) - C/C, C/T, T/T genotypes [Table 2].
| Characteristics | ABCB-CC | ABCB-CT | ABCB-TT | P |
|---|---|---|---|---|
| Number of patients | 40 | 45 | 16 | 0.061 |
| Recipient age in years (mean) | 32.22 | 33.82 | 30.06 | 0.128 |
| Donor age in years (mean) | 48 | 50.1 | 48.13 | 0.28 |
| Male: Female | 33:7 | 40:5 | 4:12 | 0.212 |
| Live donors | 60% | 80% | 68.75% | 0.741 |
| Mean creatinine (mg/dL) | 1.36 | 1.35 | 1.4 | 0.311 |
| Tacrolimus dose (mg/day) (mean±SD) | 4.5(±2.16) | 4.23(±2.17) | 3.83(±2.29) | 0.575 |
| Tacrolimus level (ng/mL) (mean±SD) | 6.46(±3.15) | 6.31(±2.15) | 7.27(±3.59) | 0.499 |
| L/D ratio (ng/mL/mg/kg/day) (median: IQR) | 74.45 | 87.75 | 116.71 | 0.26 |
| Induction treatment | 42.5% | 31.11% | 25% | 0.10 |
| Rejections | 9 (22.5%) | 9 (20%) | 4 (25%) | 0.65 |
| CNI toxicity | 3 (7.5%) | 4 (8.95) | 2 (12.5%) | 0.32 |
ABCB1: ATP binding cassette subfamily B member1
Discussion
Tacrolimus is a potent immunosuppressive drug used in solid organ transplantation. But, it has got a narrow therapeutic range, which is further complicated by wide variation in intra-individual and inter-individual bioavailability of drug. Tacrolimus is metabolized by CYP3A5 enzymes in liver and small intestine. Genetic polymorphisms in CYP3A5 are found to influence the inter-individual variability in tacrolimus trough blood levels.[2] The prevalence of CYP3A5*3*3 (CYP3A5 non-expressers) in our study was 40.6%. This is in contrast to study by Lina Quteineh et al. from France, which showed the prevalence of non-expresser genotype (CYP3A5*3*3) was 75%.[14] Another study by Ashavaid et al. from North India showed the prevalence of 40%.[13] Meaning that Indian patients have more wild-type allelic variants of CYP3A5.
In our study, we evaluated the effect of CYP3A5 genetic polymorphisms on tacrolimus daily dose requirements in a cohort of kidney transplant recipients. Our results show that carriers of at least one active allele (CYP3A5*1) needed significantly higher doses of tacrolimus compared with patients homozygous for CYP3A5*3 (CYP3A5 non-expressers). This result relies on the fact that carriers of CYP3A5*1 allele exhibit high levels of CYP3A5 expression and enzymatic activity, leading to higher daily dose requirement to achieve normal trough levels of tacrolimus. Such results have been previously replicated in the literatures concerned to this polymorphism.[56789] At 6 -month post-transplantation, patients homozygous for CYP3A5*1 needed more than threefold higher doses of tacrolimus compared with patients homozygous for the variant allele CYP3A5*3, and patients heterozygous for this polymorphism needed intermediate doses to achieve the same target trough concentration. Patients with CYP3A5*3/*3 genotype achieved fourfold higher concentration/dose (L/D) ratio compared with patients with CYP3A5*1/*1 genotype.
We also evaluated the risk of BPAR during the first month post-transplantation. We found that patients with CYP3A5*1/*3 and *1/*1 genotypes had significant higher incidence of acute graft rejection episodes compared with CYP3A5*3 homozygotes. This observation is also seen in the study by Sreeja Nair et al.[15] This observation is in agreement with the fact that carriers of the wild-type allele (CYP3A5*1) have higher levels of CYP3A5 expression, higher metabolic clearance of tacrolimus, low trough concentrations, and, therefore, acute rejection. We noticed a significant difference of tacrolimus trough concentration between carriers of at least one active allele (CYP3A5*1) and patients with CYP3A5*3/*3 genotype. Additionally, tacrolimus trough concentration of patients with at least one active allele (CYP3A5*1) was at the lower limit of the targeted trough levels and could confirm the higher risk of acute rejection in this group. Few rejection episodes occurred after the first month of transplantation (three episodes within the CYP3A5*3/*3 group), so the therapeutic tacrolimus level are more crucial during the first month after transplantation.
In our study, we were not able to get the details of HLA mismatches between donor and recipients (especially in cadaveric renal transplants), which is also an important risk factor for acute rejection. When compared to the living donor graft recipients, deceased donor graft recipients had no inceased incidence of early acute rejections (21.1% vs 23.3%) or CNI toxicity (8.5% vs 10%).
We found significant correlation between the development of tacrolimus-related nephrotoxicity and CYP3A5 genetic polymorphism. This finding could be explained by the fact that the patients with CYP3A5*3/*3 genotype had higher incidence of toxicity due to higher trough levels and higher concentration/dose ratio. We did not find an association between tacrolimus daily dose and tacrolimus-related nephrotoxicity.
We found no association between ABCB1 exon26 (3435C>T) genetic polymorphisms and tacrolimus daily requirements and concentration/dose ratio. Similar findings were observed by in the study by Lina Quteineh et al.[14] This is in agreement with most of the published data evaluating this polymorphisms.[81016] We also found no association between ABCB1 exon26 (3435C>T) genetic polymorphisms and incidence of rejection or CNI toxicity. This is in contrast to the study from North India by Ashavaid et al. who observed significantly higher tacrolimus levels and concentration/dose ratio in ABCB1 exon26 (3435C>T) homozygous mutants (T/T).
Previous study by Mohan Patel et al.[17] studied the influence of CYP3A5 polymorphism on tacrolimus drug dosing in North Indian renal allograft recipients. To our knowledge, this is the largest study to show the association between CYP3A5 genetic polymorphism and tacrolimus drug level in South Indian population and also the first study to evaluate the association between the ABCB1 genetic polymorphism in South Indian cohort. Our study is a cross-sectional study, which is relying on the retrospective data. Large-scale prospective studies will be helpful in consolidating these findings.
Conclusions
Our results confirm that CYP3A5 genetic polymorphism is an important factor in determining tacrolimus daily requirements. It was shown from our study that CYP3A5 genetic polymorphism is implicated in the risk of developing acute rejection episodes and tacrolimus-related renal toxicity. As per our study, ABCB1 polymorphisms have not been shown to influence the serum tacrolimus concentrations and dose requirements.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to acknowledge Dr. Arvind Ramanathan, Director, Enable Biolabs India Private Limited, Chennai, for providing laboratory support to conduct the experiments.
References
- The human cytochrome P450 sub-family: Transcriptional regulation, inter-individual variation and interaction networks. BiochimBiophysActa. 2007;1770:478-88.
- [Google Scholar]
- Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51:1374-81.
- [Google Scholar]
- Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383-91.
- [Google Scholar]
- The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11:773-9.
- [Google Scholar]
- Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233-5.
- [Google Scholar]
- Pharmacogenetics in solid organ transplantation: Present knowledge and future perspectives. Transplantation. 2004;78:311-5.
- [Google Scholar]
- Impact of CYP3A5 and MDR1(ABCB1) C3435T polymorphismson the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant Proc. 2005;37:1730-2.
- [Google Scholar]
- Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation. 2005;80:977-84.
- [Google Scholar]
- Tacrolimus pharmacogenetics: The CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation. 2005;79:499-502.
- [Google Scholar]
- The gut as a barrier to drug absorption: Combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet. 2001;40:159-68.
- [Google Scholar]
- G2677T and C3435T genotype and haplotype are associated with hepatic ABCB1 (MDR1) expression. J Clin Pharmacol. 2006;46:373-9.
- [Google Scholar]
- Banff '05 meeting report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy('CAN') Am J Transplant. 2007;7:518-26.
- [Google Scholar]
- Effect of gene polymorphisms on the levels of calcineurin inhibitors in Indian renal transplant. Indian J Nephrol. 2010;20:14-51.
- [Google Scholar]
- Influence of CYP3A5 genetic polymorphism on tacrolimus daily dose requirements and acute rejection in renalgraft recipients. Basic Clin Pharmacol Toxicol. 2008;103:546-52.
- [Google Scholar]
- Polymorphism of the CYP3A5 gene and its effect on tacrolimus blood level. Exp Clin Transplantation. 2015;17(Suppl 1):197-200.
- [Google Scholar]
- C3435T polymorphism in the MDR1 gene affects the enterocyte expression level of CYP3A4 rather than P-gp in recipients of living-donor liver transplantation. Pharmacogenetics. 2002;12:451-7.
- [Google Scholar]
- Influence of CYP3A5polymorphism on tacrolimus drug dosing in Indian renal allograft recipients: Initial experience. Mol Cytogenet. 2014;7(Suppl 1):P98.
- [Google Scholar]







