Translate this page into:
Role of Fine Needle Aspiration Cytology in the Rapid Diagnosis of Pulmonary Infections in Renal Allograft Recipients with Respiratory Failure
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Renal transplantation is the treatment of choice in patients with end-stage renal disease. However, allograft recipients are at a higher risk of infection due to immunosuppressive therapies. This study aimed to analyze the utility of fine needle aspiration cytology (FNAC) lung in the etiological diagnosis of pulmonary infections in renal allograft recipients with respiratory failure.
Materials and Methods:
This is a retrospective study done in post-renal transplant patients presenting with pulmonary infections and respiratory failure in the past 7 years, in whom image-guided lung FNAC was done for diagnosis.
Results:
A total of 35 renal allograft recipients presenting with respiratory failure and having focal or diffuse pulmonary opacities (lesions) on radiological imaging were subjected to lung FNAC. The mean age of the patients was 41.1 ± 11.8 years (range 19–72), with the majority being males (n = 28, 80%); six (17.1%) of them were on invasive ventilation. The diagnostic yield of FNAC in our cohort was 77.1% (27 out of 35). Microorganisms were isolated in 21 cases (60%), with Nocardia being the most common (nine cases, 25.7%), Mycobacterial tuberculosis identified in six patients (17.1%), Aspergillus in three (8.6%), and one (2.9%) each had atypical Mycobacterium, zygomycetes, and Cryptococcus. FNAC suggested viral cytopathic effect in five patients, and cytomegalovirus (CMV) quantitative polymerase chain reaction test was found positive in four of these. One case was diagnosed as adenocarcinoma lung.
Conclusion:
Lung FNAC is a useful for establishing the etiological diagnosis of pulmonary lesions in renal transplant patients with respiratory failure.
Keywords
Lung fine needle aspiration cytology (FNAC)
pulmonary infections
renal transplantation
respiratory failure
Introduction
Renal transplantation is the ideal form of kidney replacement therapy in patients with end-stage kidney disease (ESKD). The successful results of renal transplant are partially due to improved surgical and organ preservation techniques, but mainly attributed to effective immunosuppressive therapy.[12] Post-transplant infections second as the cause of death with functioning graft.[34] Intermittent use of high immunosuppression and readjustment of doses can cause opportunistic infections.[45] Although infections leading to sepsis may originate from a variety of organs, urinary tract is the commonest,[3] followed by pulmonary infections.[35]
The etiological diagnosis of pulmonary lesions in renal transplant recipients has been based on invasive procedures like bronchoscopy, thoracoscopic lung biopsies, and endobronchial ultrasound-guided biopsies (EBUS), which are challenging in patients with respiratory failure.[5678] Utility of image-guided fine needle aspiration cytology (FNAC) has been studied in nontransplant settings for diagnosis of suspected malignant lung lesions,[910] but has seldom been tried in renal transplant recipients.[11]
Suitability for percutaneous FNAC depends upon the location of the lung lesion, with those located in the pulmonary apex, medial upper lobe or periphery, or mediastinal lesions, and those abutting the pleura being accessible for FNAC. The most common complications of lung FNAC are pneumothorax, pulmonary hematoma, and hemoptysis.[12]
This study aimed to analyze the utility of image-guided FNAC lung for etiological diagnosis of pulmonary infections in renal transplant recipients with respiratory failure.
Materials and Methods
This retrospective study included renal transplant recipients presenting with history and radiological evidence of respiratory tract infection, with respiratory failure at presentation to hospital. Respiratory failure was defined as arterial PCO2 (PaCO2) greater than 45 mmHg or arterial PO2 (PaO2) less than 55 mmHg on arterial blood gas analysis, when the fraction of oxygen inspired air (FiO2) was 0.60 or greater.[13] FNAC was preferred over other modalities in patients who had radiological evidence of peripheral lesions on chest roentgenogram and/or computed tomography (CT) chest (including consolidation, cavities, pulmonary nodule, dense ground-glass opacities [GGO]). Patients on high oxygen supplementation with non-rebreather mask, noninvasive ventilation, high-flow nasal cannula, or invasive ventilation were subjected to lung FNAC as the most suitable option (as bronchoscopy was not feasible in these because of respiratory failure). Patients who were subjected to image-guided FNAC for their lung lesion over a period of 7 years (January 2014–December 2020) and completing 6 months of follow-up post-FNAC were included.
The study was approved by the institutional ethical committee, and waiver of informed consent was granted in view of retrospective data collection. The study adhered to the guidelines recommended by the Istanbul declaration. The demographic details, transplant vintage, immunosuppression details, presenting symptoms including pulmonary symptoms (like cough, respiratory congestion, fever, dyspnea, and/or hemoptysis), treatment history, requirement of oxygen supplementation, and radiological evidence of pulmonary pathology were recorded. Empirical broad-spectrum antibiotics were started in all patients at admission as per the sensitivity pattern of the local flora and later modified as per the microbiological evidence. All FNACs were done with a 22-gauge lumbar puncture needle by an interventional radiologist under CT or ultrasound guidance (as a bedside procedure with portable ultrasonography machine in sick patients, whenever needed). The smears were prepared by the pathologist who subjected the smear to rapid on-site evaluation (ROSE). The smears prepared were subjected to routine stains (May–Grunwald–Giemsa stain, Papanicolaou stain, hematoxylin and eosin stain) as well as special stains (Ziehl–Neelsen stain-20%, Ziehl–Neelsen stain- 1%, periodic acid Schiff stain, Gomori methamine silver stain). Patients with nondiagnostic FNACs underwent lung biopsies and/or bronchoscopic alveolar lavage (BAL) wherever feasible.[1415] Tacrolimus levels were maintained as per standard protocol.[1617] The patients were treated as per the etiological diagnosis. The final outcome (survival/mortality) of all these patients was recorded at 6 months from the follow-up records of these patients with the renal transplant team.
Data was described in terms of range, mean ± standard deviation (±SD), frequencies (number of cases), and relative frequencies (percentages), as appropriate. All statistical calculations were done using the Statistical Package for the Social Sciences (SPSS) 21 version (SPSS Inc., Chicago, IL, USA) statistical program for Microsoft Windows.
Results
During the study period, 126 episodes of lung infections were encountered in 108 renal allograft recipients. Lung FNAC was done in 35 of these patients who had peripheral pulmonary lesions on radiological imaging, and respiratory failure requiring high oxygen support with non-rebreather mask, non-invasive ventilation, high flow nasal cannula, or invasive ventilation. Majority of these were ABO-compatible live-related/live-emotionally related renal allograft recipients (33/35); however, two patients had undergone live-unrelated renal transplant.
The mean age of patients was 41.1 ± 11.8 years (range 19–72, median 41 with interquartile range of 33–50 years), with the majority being males (n = 28, 80%). Fever was the most common presenting symptom (n = 27, 77.1%) in these patients, followed by shortness of breath (n = 25, 71.4) and cough (n = 12, 34.3%). Six patients (17.1%) were on invasive ventilation at the time of lung FNAC.
Most cases of lung infections were encountered within the first year after renal transplant (n = 16, 45.7%), nine patients (25.7%) had them 1–3 years posttransplant, eight patients had the infections between 3 and 5 years of renal transplant (22.9%), and two (5.7%) had them more than 5 years posttransplant. Majority of these were on triple immunosuppression with prednisolone, tacrolimus, and mycophenolate (n = 32, 91.4%). Two patients, with a transplant vintage of 26 and 53 months, respectively, were on azathioprine instead of mycophenolate, and another patient with a vintage of 33 months was on dual immunosuppression with steroid and mycophenolate (calcineurin inhibitors were stopped due to calcineurin inhibitors–associated thrombotic microangiopathy, diagnosed immediately posttransplantation).
Imaging showed lung consolidation as the most common lesion (n = 19, 54.3%), followed by GGO (n = 7, 20%), cavities (n = 5, 14.3%), and lung nodules (n = 4, 11.4%). Along with the above pulmonary lesions, three patients had mediastinal lymphadenopathy, two had ascites, and one patient had mesenteric and periportal lymphadenopathy.
Etiological diagnosis on lung FNAC was made in 27 (77%) patients. Microorganisms were isolated in 21 (60%) patients. Nocardia was found in nine cases (25.7%) [Figure 1], Mycobacterium tuberculosis (MTb) in six (17.1%) [Figure 2a, b], Aspergillus in three (8.6%) [Figure 3], atypical mycobacteria in one (2.9%), Cryptococcus in one (2.9%) [Figure 4], and zygomycosis in one case (2.9%). One case of right lung consolidation with suspicion of necrotizing pneumonia on CT chest was found to have adenocarcinoma lung on FNAC (2.9%). Five patients had viral cytopathic effects seen as multinucleated giant cell on light microscopy (including one already diagnosed Nocardia positive on FNAC) and were subjected to quantitative CMV-PCR, which was positive in four of these. Eight cases on cytomorphology showed inconclusive inflammatory pathologies. Seven of these further underwent lung biopsies, and a definite pathogen could be diagnosed in four out of these, with two being MTb and one each had Nocardia and Zygomycetes. One of the patients on invasive ventilation was subjected to BAL and diagnosed as Pneumocystis jirovecii [Table 1].

- Acid-fast long filamentous bacterial colonies consistent with Nocardia (1% ZN stain- 1000×). ZN = Ziehl–Neelsen
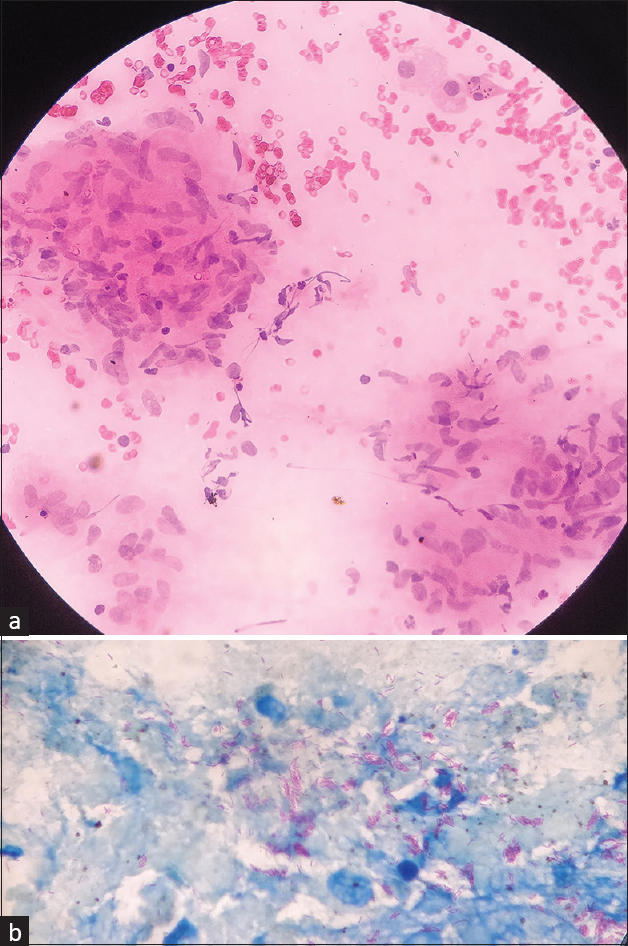
- (a) Epithelioid cell granuloma eliciting granulomatous inflammation (HE stain- 400×). (b) Beaded acid-fast bacilli consistent with Mycobacterium tuberculosis (20% ZN stain- 1000×). HE = hematoxylin and eosin, ZN = Ziehl–Neelsen

- Septate acute angle branching fungal hyphae consistent with Aspergillus (PAS stain- 400×). PAS = periodic acid Schiff

- Encapsulated round to oval yeast forms of fungus, morphologically consistent with Cryptococcus (PAS stain- 400×).PAS = periodic acid Schiff
| Etiological diagnosis Organism isolated |
Number of patients (n=35) | Risk factors | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Diagnosed on lung FNAC | Diagnosed on lung biopsy/BAL | Use of ATG as peri-transplant induction (in the last 6 months) | High-dose steroid or ATG/plasmapheresis (in the last 6 months) | Higher tacrolimus trough level at the time of presentation with infection | Survived | Mortality | Survival percentage (survived cases/total cases) | |
| Nocardia | 10 | 9 | 1 | 4 | 3 | 2 | 7 | 3 | 70% |
| Nocardia alone | 9 | 8 | 1 | 4 | 2 | 1 | 7 | 2 | 77.80% |
| Nocardia + CMV | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0% |
| Mycobacterium tuberculosis | 8 | 6 | 2 | 2 | 3 | 1 | 6 | 2 | 75% |
| Aspergillus | 3 | 3 | 0 | 1 | 1 | 0 | 2 | 1 | 66.7% |
| Zygomycetes | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0% |
| Atypical mycobacteria | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 100% |
| Cryptococcus | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 100% |
| Pneumocystis jirovecii | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 100% |
| CMV disease | 4 | 4 | 0 | 0 | 1 | 2 | 3 | 1 | 75% |
| CMV alone | 3 | 3 | 0 | 0 | 1 | 1 | 3 | 0 | 100% |
| CMV + Nocardia | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0% |
| Adenocarcinoma lung | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0% |
| Undiagnosed cases | 4 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 50% |
ATG=Anti-thymocyte globulin, BAL=Bronchoscopic alveolar lavage, CMV=Cytomegalovirus, FNAC=Fine needle aspiration cytology
Two of the patients subjected to lung FNACs developed mild hemoptysis postprocedure. Post lung biopsy mild hemoptysis occurred in one patient, and another patient had small pneumothorax. All cases were managed conservatively. No massive pneumothorax, hemothorax, or massive hemoptysis was encountered.
Overall survival at 6 months was 65.7% (23 out of total 35), with variable outcomes with respect to etiological diagnosis [Table 1].
Discussion
Renal transplantation is associated with the best survival of all solid organ transplants. However, infections continue to challenge these patients and there is a high risk of pulmonary infections with latent, opportunistic, and acquired pathogens due to immunosuppressive therapy. The frequency of post-renal transplant pulmonary infections reported in literature varies from 5% to 37%.[18] The mortality has reduced significantly in developed countries from 40% to 5% by timely diagnosis and specific management.[1920] However, tropical countries still have dismal outcome with mortality of 20%–60%, primarily due to delayed presentation and delayed diagnosis.[21] This is the pioneer study conducted to analyze the efficacy of lung FNAC in diagnosing pulmonary infections in post-renal transplant patients with respiratory failure.
In our study, more than three-fourths of patients could be successfully diagnosed for their etiologies with this minimally invasive procedure. Majority of the patients were in the first year after transplant, and most had received high-dose steroids, leukocyte-depleting antibodies, and/or plasma exchange or had higher serum calcineurin inhibitor levels in the recent past, similar to the observations reported in many other studies on pulmonary infections after transplant.[6222324]
One of the initial studies of pulmonary infections in renal transplant patients, from Cincinatti, Ohio, dating back to 1976, has reported etiological diagnosis being made on transtracheal percutaneous puncture in majority of the patients, followed by post-mortem autopsy, open lung biopsy, bronchoscopy, percutaneous needle biopsy of the lung, transbronchial biopsy, pleural biopsy, and lobectomy. Fever was the most common presenting symptom, and bacterial infections were most commonly implicated in 73.9%, with tuberculosis (TB) in 4.3%, followed by fungal, CMV, and Pneumocystis infections, and there was 41.5% overall mortality.[6]
Many studies have highlighted the utility of bronchoscopy in the rapid diagnosis of posttransplant lung infections.[7825] In a study by Young et al.[7] in immunosuppressed patients, with few of them being renal allograft recipients, BAL helped in rapid specific diagnosis in 70% by cytological examination of the fluid and in another 23% by a combination of cytology and microbiology. A recent study from North India has primarily relied on bronchoscopy as the investigative modality with 75.8% diagnostic yield, besides sputum analysis and blood culture. Almost half of their patients (45.4%) had dyspnea, but the need for oxygen supplementation does not find mention in the study. Mixed infections were most commonly encountered in their study with bacteria (45.4%), followed by tubercular and fungal (36.3% each) infections. In case of mono-microbial infection, fungal infections were the most common. The overall mortality was 22.5%.[8]
None of the above studies tried lung FNAC as the diagnostic modality, which could be a quick and safe alternative that can be performed as a bedside procedure even in patients with respiratory failure.
In 1978, Castellino and Blank[26] tried fluoroscopically guided percutaneous needle aspiration of focal pulmonary lesions in immunocompromised patients after negative or inconclusive transtracheal aspiration and other diagnostic approaches. The yield was comparable at 73%, but the complication rate was very high, with pneumothorax in 26% (half of which required tube drainage), besides a few hemoptysis episodes (3%).
Another study by de Vivo et al.[27] compared the efficacy of transtracheal aspiration or fine needle aspiration (FNA) biopsy, or both, in heart and heart–lung transplant patients for the causative diagnosis of suspected pulmonary infection, and the sensitivity was 70% and 89%, respectively. Very high yield of FNA could be attributed to its use by them only in identifiable lesions that could be used as a target; however, a high rate of pneumothorax was encountered (21%).
The spectrum of pathological yield in our study showed Nocardia as the most common lesion on lung FNAC. Risk factors like high-dose steroids or calcineurin inhibitors and a history of CMV disease were identified to increase the incidence of Nocardia in a large solid organ recipient cohort, where Nocardia affected 0.6% of all transplant recipients, with the highest incidence found in lung transplant recipients (3.5%), whereas renal allograft recipients had a relatively lower propensity (0.2%).[28]
In our study, 17% of the lung lesions were diagnosed with MTb, with a mortality of 25%. Even though TB is highly common in India, the incidence was second to Nocardia in our study, possibly because we have included only pulmonary MTb diagnosed by lung FNAC. Those diagnosed on sputum analysis and extrapulmonary TB cases were not included. As such, there is a higher frequency of TB in solid organ recipients than the general population and the involvement can be pulmonary or extrapulmonary and could be due to reactivation of latent disease or reinfection after transplant. The frequency is lower in developed countries (1.2%–6.4%) and high (5%–15%) in highly endemic nations like India and Pakistan.[1029] Two Taiwanese studies in renal transplant recipients showed 10 and 20 years incidence of 2.4%[22] and 3.8%,[23] respectively, with three fourths having pulmonary disease, and a high mortality (13%).[23] An incidence of 2.8% was reported in a Brazilian retrospective cohort, with a mortality of 12%.[24]
Fungal infections were documented in 20% cases in our study, with aspergillosis being the most common, followed by zygomycetes. One case each of Cryptococcus and P. jirovecii were also seen. The spectrum correlated with an Iranian study which also had aspergillosis as the most common fungal infection in its 8-year data on renal transplant patients.[30] However, a study from North India had the highest incidence of mucormycosis followed by aspergillosis.[31] In contrast, a Chinese study had predominantly Candida infection.[32]
Symptomatic CMV infection occurs in 20%–60% of all transplant recipients,[33] with a reported fatality rate 65%–90%.[1] We had four cases diagnosed as CMV, and three of these could be successfully treated; however, one with accompanying pulmonary nocardiosis succumbed to the disease.
This study highlights the utility of lung FNAC in the diagnosis of pulmonary infections in renal allograft recipients, especially those on oxygen supplementation. But it has its limitations. It was a retrospective study and included only those renal transplant patients who had respiratory failure and underwent FNAC for their lung lesions, so the exact incidence of lung infections after renal transplant and their respective mortality rate could not be calculated. The bacteriological cultures from blood, sputum, or pleural fluid were not included in the analysis, as the primary focus was on yield and utility of FNAC.
Conclusion
Pulmonary infections are common in renal transplant recipients, and lung FNAC can play a pivotal role in diagnosing the infectious agent in a majority of cases with percutaneously approachable lung lesions. This is a relatively convenient modality in patients with respiratory distress where bronchoscopy is challenging. Highly invasive procedures like biopsies and diagnostic thoracotomies can thus be avoided, thereby decreasing morbidity. It can effectively serve as a quick, least invasive, and cost-effective first-line diagnostic modality facilitating timely specific therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pulmonary infection in the renal transplant recipients:Analysis of the radiologic manifestations. Radiol Infect Dis. 2014;1:3-6.
- [Google Scholar]
- Research status of pulmonary infection after renal transplantation. Infect Int. 2017;6:71-6.
- [Google Scholar]
- The spectrum of infectious diseases in kidney transplantation:A review of the classification, pathogens and clinical manifestations. In Vivo. . 2015;29:415-22.
- [Google Scholar]
- Clin J Am Soc Nephrol. 2012;7:2058-70.
- Pulmonary infections after renal transplantation:A prospective study from a tropical country. Transpl Int. 2021;34:525-34.
- [Google Scholar]
- Pulmonary infections in renal transplant recipients. Hiroshima J Med Sci. 1984;33:691-6.
- [Google Scholar]
- Pulmonary infiltrates in immunocompromised patients:Diagnosis by cytological examination of bronchoalveolar lavage fluid. J Clin Pathol. 1984;37:390-7.
- [Google Scholar]
- Spectrum of pulmonary infections in renal transplant recipients in the tropics:A single centre study. Int Urol Nephrol. 2005;37:551-9.
- [Google Scholar]
- Role of image-guided fine needle aspiration cytology of lung lesions in diagnosis and primary care of patients:Experience in a Government Medical College of Eastern India. J Family Med Prim Care. 2020;9:2785-8.
- [Google Scholar]
- Computed tomogram guided fine-needle aspiration cytology of lung mass with histological correlation:A study in Eastern India. South Asian J Cancer. 2013;2:14-8.
- [Google Scholar]
- Role of fine -needle aspiration in clinical management of transplant patients. Diagn Cytopathol. 1997;17:6.
- [Google Scholar]
- Predictive complication factors for ct-guided fine needle aspiration biopsy of pulmonary lesions. Clinics. 2010;65:847-50.
- [Google Scholar]
- Respiratory failure:An overview. In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, eds. Fishman's Pulmonary Diseases and Disorders (5th). New York: McGraw Hill; 2015. p. :2152-61.
- [Google Scholar]
- Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70:515-23.
- [Google Scholar]
- Valacyclovir prophylaxis versus pre-emptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant. 2008;8:69-77.
- [Google Scholar]
- Long-term kidney transplant outcomes:role of prolonged-release tacrolimus. Transplant Proc. 2020;52:102-10.
- [Google Scholar]
- Risk factors for lung diseases after renal transplantation. J Res Med Sci. 2015;20:1127-32.
- [Google Scholar]
- Infectious complications following renal transplantation. Surg Clin North Am. 1998;78:95-112.
- [Google Scholar]
- Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-36.
- [Google Scholar]
- Pulmonary infections in the immunocompromised host. J Assoc Physicians India. 1991;39:633-6.
- [Google Scholar]
- Impact of pulmonary and extrapulmonary tuberculosis infection in kidney transplantation:A nationwide population-based study in Taiwan. Transpl Infect Dis. 2012;14:502-9.
- [Google Scholar]
- Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transpl Infect Dis. 2006;8:148-56.
- [Google Scholar]
- Clinical features and outcomes of tuberculosis in kidney transplant recipients in Brazil:A report of the last decade. Clin Transplant. 2013;27:E169-76.
- [Google Scholar]
- Utility of bronchoalveolar lavage in the diagnosis of pulmonary infections in immunosuppressed patients. J Assoc Physicians India. 2002;50:1110-4.
- [Google Scholar]
- Etiologic diagnosis of focal pulmonary infection in immunocompromised patients by fluoroscopically guided percutaneous needle aspiration. Radiology. 1979;132:563-7.
- [Google Scholar]
- Transtracheal aspiration and fine needle aspiration biopsy for the diagnosis of pulmonary infection in heart transplant patients. J Thorac Cardiovasc Surg. 1988;96:696-9.
- [Google Scholar]
- Risk factors, clinical characteristics, and outcome of nocardia infection in organ transplant recipients:A matched case-control study. Clin Infect Dis. 2007;44:1307-14.
- [Google Scholar]
- Mycobacterium tuberculosis infection in solid-organ transplant recipients:Impact and implications for management. Clin Infect Dis. 1998;27:1266-77.
- [Google Scholar]
- Pulmonary fungal infections in kidney transplant recipients:An 8-year study. Transplant Proc. 2009;41:1654-6.
- [Google Scholar]
- Fungal infection in post-renal transplant patient:Single-center experience. Indian J Pathol Microbiol. 2020;63:587-92.
- [Google Scholar]
- Pulmonary fungal infection after renal transplantation:Analysis of 40 cases. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:880-3.
- [Google Scholar]







