Translate this page into:
Is abdominal aortic calcification score a cost-effective screening tool to predict atherosclerotic carotid plaque and cardiac valvular calcification in patients with end-stage renal disease?
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abdominal aortic calcification (AAC), cardiac valvular calcification (CVC), and atherosclerotic carotid plaque (CP) are known cardiovascular risk factors. The accuracy of the AAC score in predicting CP and CVC in patients with end-stage renal disease (ESRD) is assessed in this study. Twenty-two consecutive prevalent dialysis patients (group 1) and 26 consecutive nondialysis stage V chronic kidney disease patients (group 2) were assessed for their demographic and laboratory variables. Lateral radiograph of the lumbosacral spine was used to assess the AAC score. CP and CVC were assessed using carotid sonography and echocardiogram, respectively. Prevalence of AAC, CP, and CVC in groups 1 and 2 was, respectively, 72.7%, 81.8%, and 72.7% and 76.9%, 80.8%, and 57.7%. AAC was strongly associated with CP and CVC in both groups (P < 0.001). Tests of accuracy for the AAC score as a predictor of CP and CVC showed sensitivity, specificity, positive predictive value, negative predictive value, likelihood ratio of a positive test, and likelihood ratio of a negative test, respectively, in group 1: 83%, 75%, 93%, 50%, 3.32, and 0.23 and 85%, 77%, 87%, 70%, 4.5, and 0.29, and in group 2: 90%, 95%, 83%, 69%, 3.9, 0.41, and 82%, 91%, 77%, 71%, 4.1, and 0.21. Reproducibility of the AAC score among observers was acceptable. The AAC score can predict CP and CVC with moderate accuracy in ESRD patients. However, as our study was underpowered, the findings need validation in larger, adequately powered studies.
Keywords
Cardiovascular disease
chronic hemodialysis
clinical nephrology
end-stage renal disease
vascular calcification
Introduction
Atherosclerosis in the carotid artery, evidenced by an increased carotid intima-media thickness (CIMT), is a strong, independent predictor of cardiovascular events among the general population and those with end-stage renal disease (ESRD).[12] Cardiac valvular calcification (CVC) in ESRD patients has been associated with atrial fibrillation,[3] stroke,[4] and cardiovascular disease morbidity and mortality.[5] Hence assessment for CIMT and CVC in ESRD patients becomes essential in cardiovascular risk stratification. Carotid ultrasonogram and two-dimensional echocardiogram are the commonly used imaging modalities to assess CIMT and CVC.[6] However, in a developing country like India, even these simple imaging modalities remain out of reach for most patients.
In 1986 Witteman et al.[7] reported the relationship between aortic calcification and cardiovascular mortality. More recent data also support this observation.[8] A system for quantification of calcification was described by Kauppila et al.[9] in a subgroup of participants of the Framingham heart study. It relies on lateral lumbar radiographs and the calculation of the abdominal aortic calcification (AAC) score. The predictive value of this method for cardiovascular events and mortality was validated in a large cohort of 2500 subjects in the Framingham heart study.[1011] Recently, the AAC score was shown to correlate well with electron beam computer tomography (EBCT) scores of coronary arteries in chronic hemodialysis patients.[12] AAC may also be associated with all-cause and cardiovascular mortality in ESRD.[13]
As CIMT, CVC, and AAC scores have been shown to be associated with cardiovascular events, in this study we made effort to assess the accuracy of the Abdominal aortic calcification (AAC) score in predicting CIMT and CVC. As the AAC score can be measured from a simple radiograph that costs less than 3 US dollars, this can be used as a cost-effective screening tool to predict CIMT and CVC and thereby help in cardiac risk stratification of ESRD patients.
Materials and Methods
The study was done between July and September 2009. Two groups of patients were studied. Group 1 consisted of prevalent dialysis ESRD patients on regular 3 times weekly maintenance hemodialysis, and group 2 was made up of nondialysis stage V chronic kidney disease (CKD) patients who did not require hemodialysis.
Group 1
From a total of 27 consecutive prevalent dialysis patients who attended the outpatient dialysis clinic of our hospital, four were excluded as they were receiving only once or twice weekly hemodialysis and one more patient was excluded as he refused to give informed consent for the study. CKD and ESRD were defined as per K/DOQI guidelines.[14] Patients with acute kidney injury and patients on peritoneal dialysis were not included in the study. Hence finally 22 ESRD patients formed group 1 of our study cohort.
Group 2
From a total of 33 consecutive patients with stage V nondialysis CKD, seven were excluded as they refused informed consent for the study. So finally 26 patients with stable CKD stage V who did not require dialysis formed the study cohort. Stage V CKD was defined as per K/DOQI guidelines.[14]
All study patients were from a low socioeconomic status. The study patients were predominantly from a rural background (from five villages for which our institution serves as the referral center). The average per capita income of our study patients was nearly 30 US dollars per month. Informed consent was obtained from all patients. The study was approved by the hospital's Ethics Committee.
Patients in both groups 1 and 2 were questioned about their age and smoking status by the principal investigator. Group 1 patients were questioned about the length of time they have been receiving three times weekly maintenance hemodialysis. Blood pressure and body mass index (BMI) were measured for all patients as per the standard guidelines.[1516] Serum creatinine, serum electrolytes (potassium, calcium, phosphorus, uric acid, and bicarbonate), serum alkaline phosphatase, hemoglobin, fasting blood sugar, fasting lipid profile, hemoglobin A1c (HBA1c), and serum albumin were measured in both the groups. Calcium phosphorus product was accordingly calculated. Estimated glomerular filtration rate was calculated using the modification of diet in renal disease (MDRD) formula.[17] Intact parathormone (iPTH) was measured for all the patients in both the groups using the reagent from Diagnostic Systems Laboratories, Webster, Texas. Twenty-four-hour urine was collected in both the groups and residual urine output was accordingly measured.
Assessment of of cardiac valvular calcification
Two-dimensional echocardiographic studies were performed utilizing a Sequoia 512 (Siemens, Erlangen, Germany) or Vivid 8(General Electric, Milwaukee, WI, USA) system. Digital images were acquired in the long-axis and short-axis parasternal views and the apical four- and two-chamber views. The presence of valvular calcification (aortic and mitral valve, separately) was tested visually and limited to the assessment of the presence or absence of disease without any quantification score. The same cardiologist interpreted the echocardiograms in both the groups.
Assessment of carotid plaque using CIMT
The common carotid artery intimal medial thickness (CIMT) was measured by a high-resolution B-mode (7.5-MHz transducer) echo-tracking system (Wall Track System, Neurodata). A localized echo structure encroaching into the vessel lumen was considered to be a CP if the CIMT was 50% thicker than that of neighboring sites.[1819] The presence of CP was limited to the assessment of the presence or absence of disease without any quantification score. The same cardiologist interpreted the carotid sonogram in both the groups.
Technique used to measure the AAC score
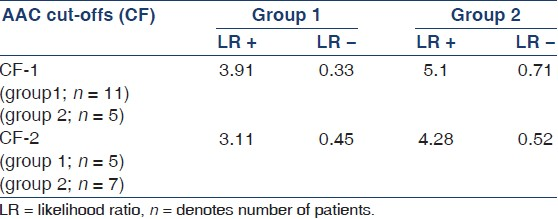
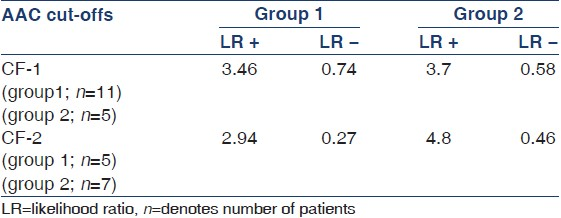
For patients in both the groups, a lateral plain radiograph of the abdomen was obtained that included the last two thoracic vertebrae and the first two sacral vertebrae. The aorta was identified as the tubular structure coursing in front of the anterior surface of the spine. A semiquantitative scoring system was utilized as suggested in the study by Kauppila et al.[9] Only the segments of abdominal aorta in front of the first to the fourth lumbar vertebrae were considered. Points were assigned from 1 to 3 (1: Small; 2: Moderate; 3: Large) according to the length of each calcified plaque identified along the anterior and posterior profile of the aorta in front of each of the lumbar vertebra taken into consideration. With this numerical grading, the score could vary from a minimum of 0 to a maximum of 24 points. A patient was defined to have AAC if the AAC score was ≥1. Furthermore, arbitrarily two cut-offs (CF) were used (CF-1; patients with AAC score between 1 and 6, and CF-2; patients with AAC score 7-24) similar to previous published studies[19] to assess the predictive accuracy of these CFs. A qualified radiologist trained in this scoring system interpreted all abdominal radiographs.
To assess intraobserver correlation in interpreting the AAC score, all abdominal radiographs in both the groups were reinterpreted by the same radiologist after a 2-month period, totally blinded about the previous interpretation results. Then, correlation was calculated comparing the present and the previous findings.
Two general physicians (GP) both trained in interpreting AAC score were involved. The first GP interpreted all the abdominal radiographs and his scoring was correlated with that of the radiologist scoring (initial findings). He was blinded regarding the findings of the radiologist and the patient characteristics. Then the second GP interpreted the abdominal radiographs and her AAC scores were correlated with those of the first GP. She was blinded regarding the findings of the first GP and the patient characteristics.
Baseline characteristics of the study patients were expressed in mean ± SD and percentage. Student's t-test and Chi-square test were used to analyze differences in baseline characteristics between patients in groups 1 and 2. Chi-square test was used to analyze the association between the presence of CP and AAC individually in both the groups and the association between the presence of CVC and AAC individually in both the groups. Parametric tests were used because data were normally distributed as tested and confirmed using tests of normality. AAC [in general AAC score from 1 to 24, and within CF-1 and CF-2] as a predictor of CP and CVC was evaluated using sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratio of a positive and negative test using standard epidemiological methods in both the groups.[20] Receiver operating characteristics (ROC) curve and area under the curve were calculated using standard methods.[20] Pearson's correlation was used to assess intra- and interobserver correlation in interpreting AAC. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS windows version 15.0 software (SPSS Inc., Chicago, Illinois). Statistical methods such as multiple regression or logistic regression could not be applied considering the small study population. To observe a prevalence of 15%, 20%, and 18% (prevalence estimates mentioned represent those observed in prior studies from global literature) for AAC, CVC, and CP, respectively, with an alpha level of 5% and 80% power, a minimum of 184 participants are required in each group. Our study with small sample size was not adequately powered.
Results
Baseline characteristics of the study population
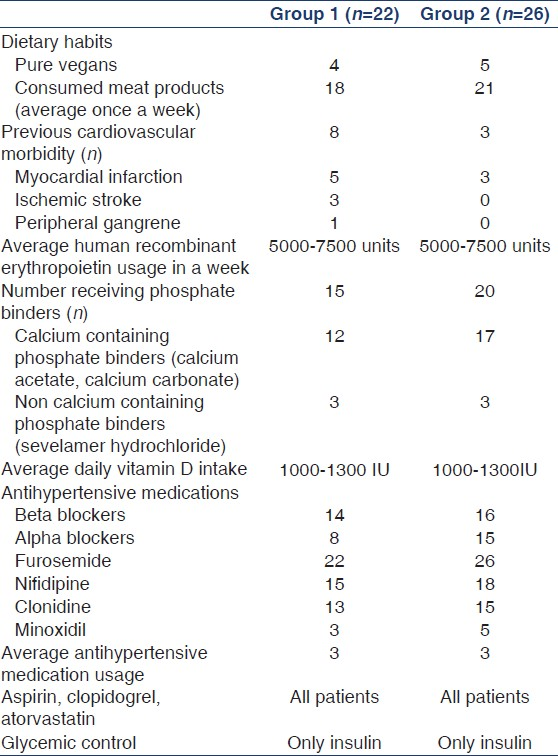
Baseline characteristics of the study population (groups 1 and 2) are shown in Table 1. Mean age of the study population in groups 1 and 2 was 51.18 ± 11.9 years and 56.69 ± 12.98 years, respectively. The mean length of time patients in group 1 were receiving dialysis was 3.1 ± 0.9 years. Dietary habits, cardiovascular comorbidities, and details of drug intake in both groups are shown in Table 2. Groups 1 and 2 were similar with respect to all patient characteristics and laboratory variables [Table 1]. The prevalence of CP in groups 1 and 2 was, respectively, 81.8% and 80.8%. The prevalence of CVC was 72.7% and 57.7% in groups 1 and 2, respectively [Table 1]. The prevalence of AAC was 72.7% and 76.9% in groups 1 and 2, respectively [Table 1].
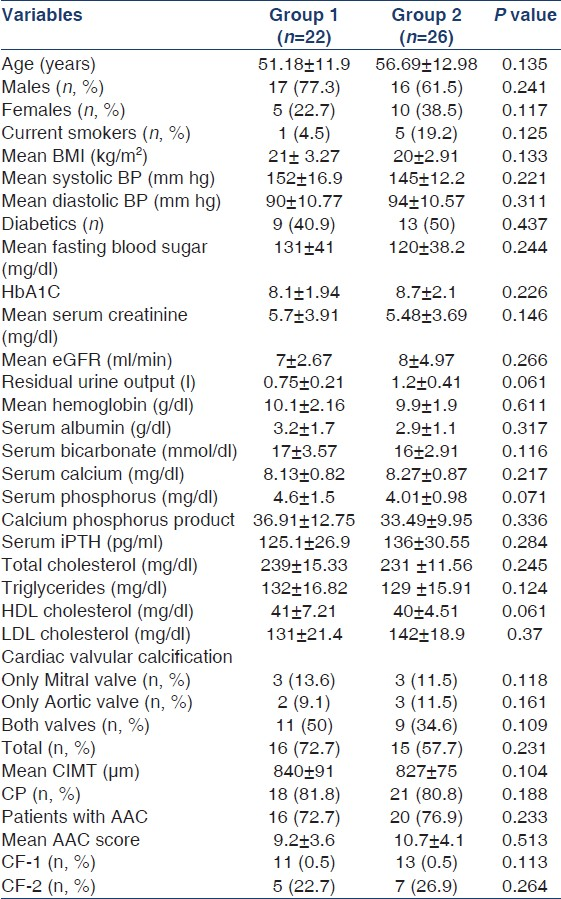
Association between CP and AAC and between CVC and AAC
Among patients with CP (group 1; n = 18 and group 2; n = 21), there was coexistent AAC in 15 patients and 19 patients in groups 1 and 2, respectively. Chi-square analysis identified a strong association between the presence of CP and AAC in both groups (group 1; P < 0.001 and group 2; P < 0.001). Similarly, among patients with CVC (group 1; n = 16, and group 2; n = 15), there was coexistent AAC in 14 patients and 13 patients in groups 1 and 2, respectively. Strong associations between CVC and AAC existed between the two groups (group 1; P < 0.001 and group 2; P < 0.001).
AAC as a screening tool to predict CP
Evaluation of AAC as a screening tool to predict CP showed a sensitivity, specificity, positive predictive value, negative predictive value, likelihood ratio of a positive test, and likelihood ratio of a negative test of 83%, 75%, 93%, 50%, 3.32, and 0.23, respectively, in group 1 and 90%, 95%, 83%, 69%, 3.9, and 0.41 in group 2, respectively. Area under the ROC curve was 84% and 81% in groups 1 and 2, respectively. The predictive accuracy of AAC within CF-1 and CF-2 in predicting CP in both the groups is shown in Table 3.
AAC as a screening tool to predict CVC
Evaluation of AAC (in general) as a screening tool to predict CVC showed a sensitivity, specificity, positive predictive value, negative predictive value, likelihood ratio of a positive test, and likelihood ratio of a negative test of 85%, 77%, 87%, 70%, 4.5%, and 0.29%, respectively, in group 1 and 82%, 91%, 77%, 71%, 4.1%, and 0.21% in group 2, respectively. Area under the ROC curve was 80% and 84% in groups 1 and 2, respectively. The predictive accuracy of AAC within CF-1 and CF-2 in predicting CVC in both the groups is shown in Table 4.
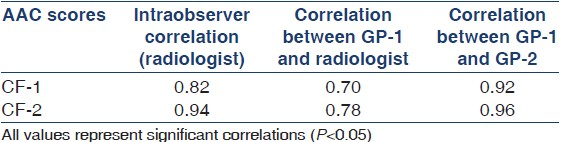
Reproducibility of AAC scores
The intra- and interobserver correlation in assessing the AAC score is depicted in Table 5. Intra-observer correlation varied between 0.82 for CF-1 and 0.94 for CF-2. Correlation between GP-1 and radiologist varied from 0.70 for CF-1 to 0.78 for CF-2. Correlation between GP-1 and GP-2 varied from 0.92 for CF-1 to 0.96 for CF-2.
Discussion
Our cross-sectional study has shown a high prevalence of AAC (group 1: 72.7% and group 2: 76.9%), CVC (group 1: 72.7% and group 2: 57.5%), and CP (group 1: 81.8% and group 2: 80.8%) in our study cohort [Table 1]. In other studies, the prevalence of CVC, AAC, and CP has been reported to be 50%,[21] 63%,[12] and 57%[22] in patients with ESRD. The reason for this high prevalence in our study cohort is probably due to the small population number. A larger population study might report comparable prevalence rates.
It is interesting to note that although a high prevalence of vascular and valvular calcification has been observed in our study, parameters such as serum calcium, serum phosphorus, calcium phosphorus product, and serum iPTH were within their normal limits. They were well within the Kidney Disease: Improving Global Outcomes (KDIGO) and KDOQI guidelines.[1423] Data from Western countries and a few East Asian countries have consistently associated disorders of mineral metabolism such as hyperphosphatemia, raised calcium phosphorus product, and hyperparathyroidism with increased prevalence of dystrophic calcification in ESRD patients.[24–26] The reason for this difference remains unclear. The study by Taniwaki et al. may give some clue to this observed difference.[27] They showed that in diabetic hemodialysis, patients’ aortic calcification could be affected by unidentified metabolic abnormalities associated with the diabetic state per se, independent of other confounding factors that occur commonly with the uremic milieu. In another study, they showed that poor glycemic control represented by higher HbA1C was a significant risk factor of peripheral artery calcification in diabetic hemodialysis patients.[28] As it can be seen, nearly 50% of our study patients (both groups 1 and 2) were diabetics with a high HbA1C [Table 1]. This could be the possible reason. Sakata et al. demonstrated the presence of carboxymethyl lysine at the site of arterial calcification and concluded that in diabetic hemodialysis patients, glycoxidation is associated with calcification of the internal thoracic artery.[29] Furthermore, it is suggested that hypoxia under hyperglycemia leads to diabetic arteriosclerosis and calcification.[30] These could be the possible mechanisms that happen in diabetic ESRD patients resulting in vascular calcification. Suresh et al. in their study involving prevalent dialysis patients from South India showed that in their cohort, serum calcium, phosphorus, and parathormone levels were predominantly within the lower limit for normal.[31] They attributed this observation to the predominance of low turnover bone disease as their cohort had significant vitamin D deficiency.[31] Though our study cohort was similar to theirs with respect to ethnic background, dietary habits, and socioeconomic status, this conclusion could not be reached in our study cohort because we do not have serum values of vitamin D in our cohort. Furthermore, recent research has reported an increased association between vascular calcification and low turnover bone disease.[3233] Calcium-based phosphate binders when used in combination with vitamin D analogs suppress serum phosphorous and serum parathormone, contributing to low turnover bone disease, and the exogenously administered calcium gets deposited and results in vascular calcification.[34] It should be noted that nearly 80% of our cohort (groups 1 and 2) were receiving treatment with calcium containing binders.
Our study has shown a strong association between AAC score, CP, and CVC. The study by Bellasi et al. had shown a strong association between AAC score and calcium deposition in the coronary arteries as measured by electron beam-computed tomogram.[12] To the best of our knowledge, there are no comparable studies that have evaluated the association between AAC score, CVC, and CP. Our study is probably the first of its kind.
Our analysis on the accuracy of AAC scores in predicting CP and CVC showed interesting findings. From the LR+, LR, and area under the ROC curve values, we may conclude that the AAC score in its entire range (in general, CF-1 and CF-2) has a moderate accuracy in predicting CP and CVC in both groups.[20] Our assessment of reproducibility of the AAC score showed good intra- and interobserver correlation [Table 5]. The correlation between GP-1 and GP-2 was good and also GP-1 had appreciable correlation with the assessment by the radiologist although the strength of this correlation was lesser when compared with the correlation between the two GPs. The main objective of this study was to devise a cost-effective screening tool to be of use in a resource-poor community setting to predict CP and CVC in ESRD patients. As there is a fairly good intra- and interobserver correlation in assessing the AAC score, we may conclude that AAC score can be useful as a screening tool to predict CP and CVC in a resource-poor setting. Furthermore, this screening tool may be used by any GP having knowledge in its interpretation.
The CORD study has observed that this simple cost-effective tool was less expensive than most available imaging modalities that can detect AAC and hence had proposed its use in the cardiovascular risk stratification and pretransplant workup of ESRD patients.[35] Our study findings strongly support this idea.
Small population size is an important limitation of our study. Larger population studies with a similar concept have to be performed before this idea can be put to clinical use. This being a cross-sectional study, it was not possible to follow up if these observations remain valid over time. Furthermore, inflammatory markers and their association with vascular calcification, an association that has been well reported in the literature, were not assessed in our study. The application of our study findings to day-to-day clinical practice relies on the ability of physicians to interpret the AAC score. Reasonable physician training in interpreting the AAC score is required before this concept could be put to clinical use.
Conclusion
It can be inferred from the sample size calculations we had mentioned in the statistical analysis section that our study with its small sample size was not adequately powered to answer all the objectives of our study. Hence, the validity of the estimates that we report cannot be relied upon with certainty and needs confirmation in larger population studies. However, via this study we aim to propose the utility of the AAC score as a possibly effective, clinically applicable screening tool that may be used for cardiovascular risk stratification of patients with ESRD and to stimulate further research with this concept. It is for adequately powered, larger population studies to answer if our findings can be reproduced and if indeed AAC score makes its way into clinical practice as a screening tool as proposed by us.
Source of Support: Educational grant from Tamilnadu Kidney Research foundation (TANKER), Chennai, India
Conflict of Interest: None declared
References
- Carotid intima-media thickness as a measure of vascular target organ damage. Curr Hypertens Rep. 2000;2:71-7.
- [Google Scholar]
- Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12:2458-64.
- [Google Scholar]
- Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis. 2004;173:291-4.
- [Google Scholar]
- Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374-9.
- [Google Scholar]
- Mitral annular calcification predicts cardiovascular morbidity and mortality: The Framingham Heart Study. Circulation. 2003;107:1492-6.
- [Google Scholar]
- American Society of Echocardiography Report.Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: A report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med. 2006;11:201-11.
- [Google Scholar]
- Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120-2.
- [Google Scholar]
- On Behalf of the Vascular Calcification Work Group.Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572-9.
- [Google Scholar]
- New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-years follow-up study. Atherosclerosis. 1997;132:245-50.
- [Google Scholar]
- Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529-34.
- [Google Scholar]
- Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: The Framingham heart study. Am Heart J. 2002;144:733-9.
- [Google Scholar]
- Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int. 2006;70:1623-8.
- [Google Scholar]
- Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417-25.
- [Google Scholar]
- K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266.
- [Google Scholar]
- National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7) JAMA. 2003;289:2560-71.
- [Google Scholar]
- Jeffrey SF, Eleftheria MF, Kasper DL, Braunwald E, Fauci AS, eds. Harrison's principles of internal medicine Vol 1. (16th ed). New York: Mc Graw Hill Inc; 2005. p. :422-3.
- Chronic kidney disease epidemiology collaboration.Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-54.
- [Google Scholar]
- Cardiac and arterial interactions in endstage renal disease. Kidney Int. 1996;l50:600-8.
- [Google Scholar]
- Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909-18.
- [Google Scholar]
- Diagnostic testing. In: Greenberg RS, ed. Medical Epidemiology. Vol 2. New York: McGraw-Hill; 2005. p. :3291-6.
- [Google Scholar]
- Crosssectional association of kidney function with valvular and annular calcification: The Framingham heart study. J Am Soc Nephrol. 2006;17:521-7.
- [Google Scholar]
- Arterial calcifications, arterial stiffness, and cardiovascular risk in endstage renal disease. Hypertension. 2001;38:938-42.
- [Google Scholar]
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKDMBD) Kidney Int Suppl. 2009;113:S1-130.
- [Google Scholar]
- Pathophysiological mechanisms of vascular calcification in endstage renal disease. Kidney Int. 2001;60:472-9.
- [Google Scholar]
- US Renal Data System. In: USRDS 1998 Annual Data Report. Bethesda: The National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases;; 1999.
- [Google Scholar]
- Aortic calcification in haemodialysis patients with diabetes mellitus. Nephrol Dial Transplant. 2005;20:2472-8.
- [Google Scholar]
- Different risk factors for peripheral vascular calcification between diabetic and nondiabetic haemodialysis patients-importance of glycaemic control. Diabetologia. 2002;45:1446-8.
- [Google Scholar]
- Calcification of the medial layer of the internal thoracic artery in diabetic patients: Relevance of glycoxidation. J Vasc Res. 2003;40:567-74.
- [Google Scholar]
- Parathyroid hormone and biochemical profile in chronic kidney disease patients in South India. Hemodial Int. 2005;9:63-7.
- [Google Scholar]
- Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J Bone Miner Res. 2010;25:499-504.
- [Google Scholar]
- Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf). 2009;71:644-51.
- [Google Scholar]
- Low turnover bone disease in patients with chronic renal disease. Med Pregl. 2007;60:21-4.
- [Google Scholar]
- Abdominal aortic calcification in dialysis patients: Results of the CORD study. Nephrol Dial Transplant. 2008;23:4009-15.
- [Google Scholar]







