Translate this page into:
Kidney Transplantation with ABO-Incompatible Donors: A Comparison with Matched ABO Compatible Donor Transplants
Address for correspondence: Dr Aniketh Prabhakar, Department of Nephrology, Mulijibhai Patel Urological Hospital, Nadiad - 387 001, Gujarat, India. E-mail: anikethprabhakar@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
ABO-incompatible kidney transplantation (ABOiKTx) expands the living donor pool. There is limited long-term outcome data from India especially in comparison with ABO-compatible kidney transplantation (ABOcKTx). Here we report outcomes of the first 100 ABOiKTx compared to ABOcKTx from our center.
Methods:
Between August 2013 and December 2019, 100 consecutive ABOiKTx were compared with 100 ABOcKTx done during the same period.Controls were matched for age, donor characteristics, HLA mismatches, and date of transplantation.
Results:
Mean (SD) follow up period was 25.9 ± 20.5 and 27.2 ± 20.6 months in ABOi and ABOcKTx respectively. Patient survival at 1 and 5 years post-transplant was 93.3 and 73.5% vs. 95.4 and 93% (P = 0.03), while graft survival rates were 85 and 60% vs. 93.1 and 83% in ABOi and ABOcKTx respectively (P = 0.03). The incidence of antibody-mediated rejections was 15% vs. 4%, and that of T-cell-mediated rejections was 10 vs. 12% respectively. Infections, malignancies, and surgical complications were similar. Level of anti ABO titers, HLA mismatches, recipient age, donor age, and presence of diabetes did not impact graft survival amongst ABOiKTx. The predicted survival and incidence of acute rejections and infections in the later 50 ABOiKTx transplants were better than the first 50 ABOiKTx when compared to their respective controls.
Conclusion:
Outcomes of ABOiKTx were inferior to ABOcKTx but tends to improve as more experience is gained. Incidence of ABMR was higher but infections and surgical complications were comparable. This data provides evidence that ABOiKTx is viable option for those without a ABO compatible donor.
Keywords
ABO incompatible
outcomes
renal transplant
survival
Introduction
Many suitable living related donors were rejected (upto 45%) in the past in view of blood group incompatibility.[1] In the last two decades, with fine-tuning of desensitization protocols and effective immunosuppression, ABO-incompatible kidney transplant (ABOiKTx) has become an alternative option to expand the living donor pool.[2] Concern still exists, however, that ABOiKTx is associated with an increased risk of rejections, infections, and surgical complications, partly owing to the effects of intensified immunosuppression and plasmapheresis.[3]
Recently Indian society of organ transplant has given guidelines for performing ABOiKTx.[4] There is limited short-term data from India;[56] however, long-term data is lacking. ABOiKTx program was initiated at our institute in 2013. Here we report the outcomes of our first 100 ABOiKTx performed between August 2013 and December 2019 compared to ABOcKTx performed during the same period.
Methods
This is a retrospective comparative study of outcomes of ABOiKTx with age, HLA mismatches, and donor characteristics matched ABOcKTx performed during the same period. The controls were chosen using the same transplant list which did not include information on transplantation results. The controls were selected based on following criteria ranked in order of importance: date of transplants (±1 month), age (±5 years), recipient gender, dialysis vintage, recipient hypertension, diabetes, ischemic heart disease, repeat transplants, pregnancy status of female recipients and HLA mismatches on A, B, DR locus (±2).
Protocol for ABO-incompatible kidney transplant
Once a suitable donor was identified, recipient serum was tested for IgG antibody titer against donor ABO blood group antigens. This was done using column agglutination technology with Low-Ionic-Strength (Biorad Laboratories). The cassettes used were anti-human globulin type. A cutoff of titers ≤1:1024 was fixed. All prospective donors underwent standard investigations after a detailed history and physical examination. Crossmatch was performed by complement-dependent cytotoxicity (CDC). HLA typing for donor and recipient was done at A/B/DR loci by next-generation sequencing.
Desensitization and immunosuppression [Figure 1]
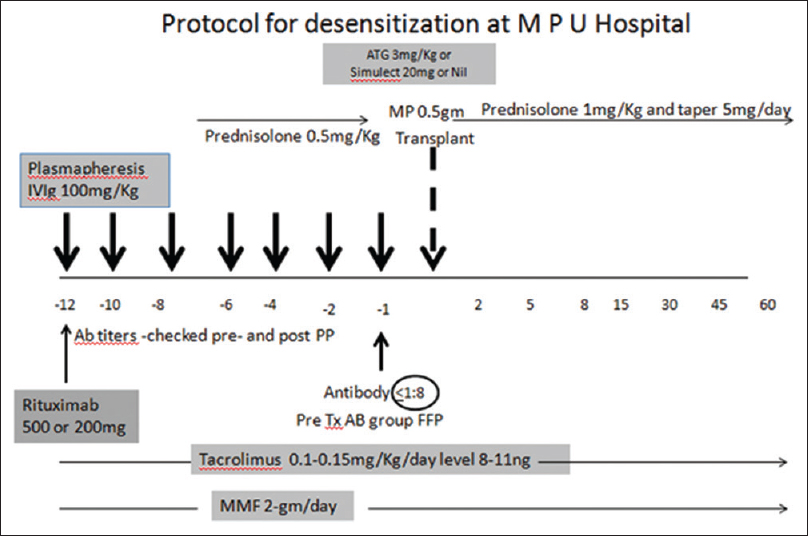
- Desensitization protocol used
All the patients underwent desensitization with one dose of anti-CD-20 antibody (Rituximab) at a dose of 500mg or 200mg based on physician discretion, given on day -15 to -20 of transplant. Patients received oral tacrolimus 0.15 mg/kg in two divided doses (aimed trough level 8-11 ng/ml), mycophenolate mofetil (MMF) 2gm/day in 2 divided doses and prednisolone at 0.5mg/kg started from day -10. Patients were initiated on plasmapheresis sessions several days prior to transplantation based on antibody titer and was continued till the titer fell below 1:8. Inj methyl prednisolone 1 g was given to all patients just before surgery. Choice of induction [Nil/InjBasilixmab/Inj Anti thymocyte globulin (ATG)] was as per physician preference. Dose of basiliximab used was 20 mg in 2 doses on day 0 and day 4, Inj ATG was used at a dose of 3mg/kg. Tacrolimus 0.15 mg/kg (trough level of 8–12ng/mL), MMF 2 gm per day and prednisolone 20mg (with gradual tapering) were continued as maintenance immunosuppresion. By 3 months prednisolone was tapered down to 7.5 mg per day, Tacrolimus was continued to maintain a trough level of 6 to 10ng/mLand MMF was reduced to 1g per day. Valgancyclovir as anti cytomegalovirus (CMV) prophylaxis was given to all patients for 100 days. All our patients had a D+/R+ serostatus for CMV.
Protocol for ABO compatible kidney transplant
Patients received oral tacrolimus 0.15 mg/kg in two divided doses (aimed trough level 8–11 ng/mL), mycophenolate mofetil (MMF) 2gm/day in two divided doses from day –2. Choice of induction therapy (Nil/Inj Basilixmab/Inj ATG) was as per physician preference. Maintenance therapy was similar to ABOiKTx and anti CMV prophylaxis was given only to patients who received ATG. All our patients had a D+/R+ serostatus.
All patients were followed up in transplant clinic initially thrice weekly for 2 weeks, then twice a week for 2 months and less frequently thereafter. Patients missing their follow-up date were traced actively with phone calls and their status was ascertained by creatinine measurement at a local lab. Posttransplant ABO titers were done only for graft dysfunction.
Baseline characteristics were assessed from clinical records. Follow-up data were collected from post-kidney transplant OPD facilities. Graft function was assessed by serial measurement of serum creatinine and at one, three, six and twelve months and every six months thereafter. Data was collected till February 2020, at which time graft survival and patient survival was assessed. Graft failure was defined by the need to resume dialysis permanently and death of the patient. Graft biopsies were performed when creatinine increased by 25% of the baseline and the cause was not obvious. Histology samples of transplant kidney biopsies were scored according to latest Banff criteria applicable at the time of biopsy. Infectious complications recorded comprised of bacterial infections, tuberculosis, cytomegalovirus disease (defined by CMV replication and clinical symptoms), BK virus (defined by BKViremia), herpes zoster, pneumocystis pneumonia and fungal infections. Non-infectious complications recorded included malignancies and surgical complications.
Data are expressed as mean ± standard deviation (range) and Median. Group comparison was performed with Student's t-test for continuous data and Fisher's exact test for discrete data. Kaplan–Meier survival analysis was compared by the log-rank test. Statistical significance was assumed at a P value of <0.05. A cox proportional hazard models was used to examine risk factors for patient survival and results were expressed as Hazard ratios with 95% Confidence intervals and P values. The statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software version 23.0 (IBM, Chicago, IL, USA).
Results
As shown in Table 1 recipient and donor characteristics of the two cohorts did not significantly differ in age, gender distribution, proportion of related donors, proportion of pre-emptive transplantation, dialysis vintage or presence of hypertension, diabetes or coronary artery disease. Both groups had comparable HLAmismatches, number of pregnancies amongst female recipients and second transplants.
| Character | ABO I | ABO C | P |
|---|---|---|---|
| Mean Age of Recipients (range) in years | 40.89 (17-61) | 40.81 (17-68) | 0.30 |
| Mean Age of Donors (range) in years | 48 (24-69) | 48.3 (26-66) | 1.0 |
| Recipients (Male:Female) | 85/15 | 84/16 | 1.0 |
| Donors (Male:Female) | 81/19 | 84/16 | 0.70 |
| Donors (Related/Emotionally Related)* | 54/46 | 59/41 | 0.50 |
| Pre emptiveTransplants | 11 | 12 | 1.0 |
| Dialysis vintage (Range) in days | 173 (0-360) | 165 (0-390) | 0.43 |
| Recipient Hypertension | 97 | 96 | 1.0 |
| Diabetes | 26 | 23 | 0.74 |
| Ischemic Heart Disease | 13 | 11 | 0.82 |
| Repeat transplants | 12 | 11 | 1.0 |
| Pregnancy | 14/15 | 13/16 | 0.64 |
| CMV D+/R+ | 100 | 100 | 1.0 |
| ≤3 HLA Mismatch | 58 | 65 | 0.20 |
| >3 HLA mismatch | 42 | 35 | 0.20 |
*Emotionally related includes wives, uncles, aunties and in-laws CMV=Cytomegalovirus HLA=Human Leukocyte Antigen
Blood group distribution amongst recipients and their donors for ABOiKTx are described in Figure 2. B group (Donor) to O group (recipient) (30%) was the most frequent pairing. Frequency of different ABO titers vs. the number of plasmapheresis sessions needed are enumerated in Figure 3. Single filter plasmapheresis was used in 95 patients, Double filtration plasmapheresis was used in 3 patients and 2 patients underwent antibody removal by Immunoadsorption filter. This decision was based on access to techniques and patients ability to bear the cost of these technologies. During the first 2 years after noticing increased infectious complications an active change in pre transplant induction immunosuppression practices were made [Table 2]. Usage of ATG was reduced and several transplants were performed without induction agent at the time of transplantation.

- Blood group distributions

- Mean plasmapheresis sessions Vs Titers (No. of patients)
| Characters | First 50 ABOi(%) | First 50 ABOc(%) | P | Last 50 ABOi(%) | Last 50 ABOc(%) | P |
|---|---|---|---|---|---|---|
| Induction Immunosuppression | ||||||
| Anti Thymocyte Globulin | 14 | 21 | 0.10 | 1 | 33 | <0.01 |
| Basilixmab | 18 | 17 | 0.5 | 24 | 9 | <0.01 |
| Nil | 18 | 12 | 0.13 | 25 | 8 | <0.01 |
| Mean creatinine on last Follow up | 1.23 (0.36) | 1.28 (0.45) | 0.12 | 1.28 (0.39) | 1.37 (0.52) | 0.08 |
| Graft Loss | 16 | 6 | 0.014 | 10 | 4 | 0.07 |
| Death | 9 | 2 | 0.02 | 3 | 2 | 0.5 |
| Biopsy Proven Acute rejections | 15 | 10 | 0.17 | 13 | 8 | 0.16 |
| Infections | ||||||
| Urinary tract Infections | 29 | 25 | 0.27 | 20 | 35 | 0.002 |
| Cytomegalovirus | 3 | 5 | 0.35 | 0 | 1 | 0.5 |
| Fungal | 2 | 2 | 1.0 | 1 | 0 | 0.5 |
Patient survival and graft survival
The mean follow-up of the ABOiKTx cohort versus ABOcKTx cohort was 25.9 (20.5) and 27.2 (20.6) months respectively [Table 3]. Kaplan–Meier estimated median patient survival differed significantly, 93.3%, 85% in ABOiKTx at 1and 5 years versus 95.4%, 93% in ABOcKTx At 1 and 5 years respectively [Figure 4] (P = 0.03). Infections and cardiovascular disease were the leading cause of death. Estimated graft survival was 73.5% and 60% in ABOiKTx at 1 and 5 years, respectively, and 93% and 83% in ABOcKTx at 1 and 5 years, respectively [Figure 4]. The Causes of graft loss were mainly long-term chronic rejections in both groups. Hemolytic uremic syndrome, graft thrombosis was less frequent and occurred early in follow-up [Table 3].
| Factors | ABO I | ABO C | P |
|---|---|---|---|
| Mean Follow Up (SD) in months | 25.9 (±20.5) | 27.2(±20.6) | 0.8 |
| Mean S Cr. At last follow up (mg/dl) | 1.26±0.37 | 1.34±0.60 | <0.01 |
| Graft Loss | 26/100 | 10/100 | <0.01 |
| Deaths | 12/100 | 4/100 | 0.06 |
| Cause of Graft loss | |||
| Graft artery thrombosis | 1 | 0 | 1 |
| HUS | 1 | 0 | 1 |
| Chronic Rejection | 12 | 6 | 0.21 |
| Uncertain | 6 | 1 | 0.11 |
| Death | 6 | 1 | 0.11 |
| BKV | 0 | 2 | 0.5 |
| Cause of Death | |||
| Anastomotic leak | 2 | 0 | 0.5 |
| Acute MI | 2 | 1 | 1.0 |
| Sepsis | 3 | 1 | 0.62 |
| RTA | 1 | 0 | 1.0 |
| Unknown | 3 | 1 | 0.62 |
| Malignancy | 1 | 1 | 1.0 |
HUS: Hemolytic Uremic Syndrome, BKV: BK Virus, MI: Myocardial Infarction, RTA: Road Traffic Accident

- Kaplan-meier analysis of patient and graft survival after kidney transplant. (a) Graft survival (in months) between All 100 ABOi (Green) and ABOc (Blue) in the study (P = 0.03) which is lower in the incompatible group. (b) Patient Survival (in months) between All 100 ABOi (Green) and ABOc (Blue) in the study (P = 0.03) which is lower in the incompatible group. (c) Graft survival amongst latest 50 Transplants inABOi (Green) and ABOc (Blue) transplants (P = 0.09). (d) Graft survival amongst First 50 Transplants in ABOi (Green) and ABOc (Blue) transplants (P = 0.008). In the graphs above the number of observations can be seen on the timeline
Rejection episodes [Table 4] were seen in 28% patients in ABOi-KTx group and 18% of ABOc-KTx controls. Antibody mediated rejection was seen in 15% of ABOi-KTx cohort versus 4% in ABOc-KTx cohort and was significantly higher (P < 0.01). T cell mediated rejections in 10 and 12% (P = 0.4) respectively and were comparable in both groups.
| Character | ABO I | ABO C | P | ||
|---|---|---|---|---|---|
| Total rejections | 28 | 18 | 0.06 | ||
| ABMR | 15 | 4 | 0.01 | ||
| Time line | No. | Recovery from rejection(%) | No. | Recovery from rejection(%) | |
| 0-1 month | 12 | 4 (33) | 0 | 0 | |
| months | 2 | 0 | 2 | 1 (50) | |
| >6 months | 1 | 0 | 2 | 0 | |
| ACR | 10 | 12 | 0.4 | ||
| Time line | No. | Recovery from rejection(%) | No. | Recovery from rejection(%) | |
| 0-1 Months | 2 | 2 (100) | 7 | 7 (100) | |
| 1-6 Months | 5 | 3 (60) | 4 | 2 (50) | |
| >6 months | 3 | 2 (66) | 1 | 0 | |
| Mixed | 3 | 3 | 1.0 | ||
| Time line | No. | Recovery from rejection(%) | No. | Recovery from rejection(%) | |
| 0-1 Months | 1 | 0 | 0 | 0 | |
| 1-6 Months | 1 | 0 | 2 | 2 (100) | |
| >6 months | 1 | 0 | 1 | 0 | |
ABMR: Antibody Mediated Rejections, ACR: Acute Cellular Rejections
In both groups the most common infection [Table 5] was UTI. (49% in ABOi-KTx cohort versus 60% ABOc-KTx cohort, P = 0.12) There was no difference in the incidence of Tuberculosis, Cytomegalovirus, BK Virus, herpes zoster, fungal and pneumocystis infections between the two groups. Each group had 1 malignancy [Table 5]. In the ABOiKTx patient developed non melanoma skin cancer 2 years after transplant and the patient in the ABOcKTx patient developed Post transplant lymphoproliferative disorder 1 year following transplant. Surgical complications comprised of vascular anastomotic leak (2%) and graft artery thrombosis (1%) inABOi-KTx cohort and none of such were seen in the ABOc-KTx cohort. Other complications are as enumerated in Table 5.
| Characters Infections | ABO i | ABO c | P | ||
|---|---|---|---|---|---|
| <3 months | >3 months | <3 months | >3 months | ||
| Urinary tract Infections | 47 | 2 | 55 | 5 | 0.15 |
| PCP | 1 | 1 | 0 | 0 | 0.5 |
| Tuberculosis | 1 | 2 | 2 | 3 | 0.72 |
| Herpes zoster | 0 | 2 | 0 | 1 | 1.0 |
| CMV | 2 | 1 | 4 | 2 | 0.49 |
| BKV | 0 | 6 | 0 | 6 | 1.0 |
| Fungal infection | 2 | 1 | 2 | 0 | 1.0 |
| Malignancy | 1 | 1 | 1.0 | ||
| Surgical Complications | |||||
| Uretric leak | 2 | 1 | 1.0 | ||
| Anastomotic leak | 2 | 0 | 0.5 | ||
| Graft Artery thrombosis | 1 | 0 | 1.0 | ||
| Lymphocoele | 2 | 4 | 0.68 | ||
| TRAS | 5 | 4 | 1.0 | ||
| Hematoma | 0 | 1 | 1 | ||
PCP: Pneumocystis Carinii Pneumonia, CMV: Cytomegalovirus, BK Virus, Transplant renal artery stenosis
Factors like presence of diabetes, recipient age, donor age, blood group type, and anti blood group antibody titers were analyzed for their bearing on the graft survival of the ABOiKTx [Table 6]. None of the above mentioned factors were found to impact graft survival. A cox proportional hazards model [Table 7] used to study the impact of variables like recipient/donor age, induction agent, biopsy proven acute rejections, presence of ABO incompatibility, haplomatch status, recipient diabetes on graft survival revealed that ABO incompatibility (HR 2.309, 95% CI (1.121- 4.759), P. 023) and rejection episodes (HR 4.890, 95% CI (2.481-9638), P < 0.001) were major hazards.
| Character | No of Patients | No of Graft Surviving (%) | P |
|---|---|---|---|
| Diabetes | |||
| Yes | 26 | 18 (69) | 0.54 |
| No | 74 | 56 (75) | |
| Donor Age | |||
| <55 years | 71 | 56 (78.8) | 0.11 |
| >55 years | 29 | 18 (62.0) | |
| HLA Mismatches | |||
| <3 | 58 | 44 (75.8) | 0.63 |
| >3 | 42 | 30 (71.4) | |
| Recipient Age | |||
| <40 | 42 | 34 (80.9) | 0.0 |
| >40 | 58 | 40 (68.9) | |
| Blood group | |||
| O | 53 | 38 (71.6) | 0.63 |
| Non O | 47 | 36 (76.5) | |
| Titre | |||
| ≤1:64 | 58 | 45 (77.5) | 0.13 |
| >1:64 | 42 | 29 (69.0) |
HLA: Human Leukocyte Antigen
The learning curve
Over 6 years we have been fine tuning our programme. The changes included a) reduced use of ATG as induction agent. [Table 2], b) Use of lower doses of rituximab in the last 50 patients i.e., change from 500 mg to 200 mg. These changes resulted in better graft survival in the latest 50 ABO iKTX compared to the first 50 ABOi KTx counterparts. [Figure 4]. Biopsy-proven acute rejection episodes were lesser and the differences in graft losses (P = 0.014 vs. 0.07) and Deaths (0.02 vs 0.5) had become less significant between the first 50 and last 50 ABOiKTx when compared to their respective controls. Infections including UTI, cytomegalovirus, and fungal infections also showed reduced numbers.
Discussion
This study shows that outcomes of ABOiKTx were inferior ABOcKTx. Patient and graft survival was significantly different; however, the differences were diminishing as more experience is gained. There was a higher proportion of ABMR amongst the ABOiKTx. Infections, malignancies, and surgical complications were comparable between the groups.
These results add to previous information about long-term outcome of ABOiKTx performed in India. The longer follow-up and depiction of changing patterns over time are strengths of this study. The differences in our graft and patient survival between ABOi and ABOc groups is in concordance to single-center studies of Okumi et al.,[7] Montgomery,[8] and Jha.[5] The European registry data from Opelz[9] also show a significant difference in early patient and graft survival. The recently published 2 meta-analyses by de weerd et al.[3] and Scurt et al.[10] also showed lower graft and patient survival in ABO-incompatible transplants. However, several centers[1112] have shown comparable results to compatible transplants.
Over all rate of biopsy proven acute rejections were similar in ABOiKTx and ABOc-KTx. However, there was a significantly higher number of antibody-mediated rejections. Similar data have been reported in other studies and meta-analysis.[313] The most common cause of graft losses were antibody-mediated rejections in all the studies.[5813] This accounted for 46% of losses in our study. It was unfortunate in our series that we had 3 nonimmunological, noninfectious causes of early graft loss which included 2 vascular anastomosis leaks and 1 graft artery thrombosis which contributed significantly to overall poor graft survival in our patients. In other studies, the main cause of death was cardiovascular events, but in our series it was equally distributed between cardiovascular disease, infections, and surgical complications.
In our study the infections and surgical complications were also not significantly increased as detailed in Table 6. This is in contrast to the meta-analysis by de weered et al.[3] and Korean studies,[14] which indicate high rates of infection. Both the studies from the Indian subcontinent[511] comparing outcomes between ABOcKTx and ABOiKTx showed no increase in infection rates between groups with good graft survival. This may be attributed to reduced use of ATG as induction. Bleeding and surgical complications were similar in both our groups. This was also the experience of Shin[14] but not of others[3712] The lowered rates may be attributed to use of fresh frozen plasma as replacement in all our antibody removal sessions.
In an attempt to determine the factors that may have contributed to the poor outcomes in the ABOiKTx, presence of diabetes, recipient age donor age anti-blood group antibody titer level and recipient blood group were analyzed for their impact on outcomes but no factor could be ascertained to significantly affect graft survival [Table 2]. Most studies have compared[315] impact of technique of antibody removal with outcomes. In our study few patients underwent double filtration and immunoadsorption; hence, such a comparison was not possible. In view of the large difference in mortality between ABOiKTx and ABOcKTx, a multivariate analysis was done to find out the significance of risk factors like recipient age, donor age, HLA match, ABO incompatibility, induction agent, rejection episodes, urinary tract infection, and graft survival. Results [Table 7] revealed that only older recipients and recipient who also had graft loss had increased mortality. Our results have improved over time; improved graft survival, lesser rejections, lesser infections. This has also been the experience of other centers.[7] We feel that avoiding ATG at the time of transplantation significantly reduced the infectious complications, which in turn translated to better survival.
| Risk Factors | P | Hazard ratio | 95.0% CI for Hazard Ratio | |
|---|---|---|---|---|
| Lower | Upper | |||
| Recipient Age | 0.025 | 1.097 | 1.012 | 1.190 |
| Donor Age | 0.438 | 0.967 | 0.889 | 1.052 |
| Haplomatch | 0.784 | 0.902 | 0.43 | 1.89 |
| Induction | 0.766 | 1.063 | 0.711 | 1.59 |
| Recipient Diabetes | 0.552 | 1.535 | 0.374 | 6.308 |
| Rejection episodes | 0.740 | 1.253 | 0.331 | 4.742 |
| Graft Survival | 0.000 | 0.835 | 0.770 | 0.905 |
| ABOincompatibility | 0.657 | 1.433 | 0.293 | 6.996 |
| Urinary tract infections episodes | 0.344 | 2.177 | 0.435 | 10.909 |
The limitations of this study are lack of a standard protocol for immunosuppression amongst both groups. Further, donor-specific antibody assay could not be performed in patients who had antibody-mediated rejections, making it difficult to differentiate the impact of ABO antibody vs. anti HLA antibody. Also the observational nature of the study gives way to high bias.
In conclusion, the results of ABOiKTx are inferior when compared to the ABOcKTx but continues to improve as we gain more experience. Presently, deceased donor and paired kidney exchange programs are unable to meet the demands of growing number of end-stage renal disease patients. ABO-incompatible renal transplant remains a good option to patients without a suitable blood group matched donor, albeit with a slightly inferior outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I thank my teachers and colleagues for their support and help in preparing this manuscript. I especially thank Dr. Atul V Muley Consultant Nephrologist and Clinical Epidemiologist, Pune for his valuable guidance with statistical analysis. I also thank my patients for their cooperation and trust.
References
- Medical and non-medical factors that affect voluntary living-related kidney donation: A single-center study. Indian J Nephrol. 2011;21:14-20.
- [Google Scholar]
- Strategies to overcome the ABO barrier in kidney transplantation. Nat Rev Nephrol. 2015;11:732-47.
- [Google Scholar]
- ABO-incompatible kidney transplant outcomes: A meta-analysis. Clin J Am Soc Nephrol. 2018;13:1234-43.
- [Google Scholar]
- ABO-incompatible kidney transplantation: Indian working group recommendations. Indian J Transplant. 2019;13:252-8.
- [Google Scholar]
- ABO-incompatible renal transplantation in developing world - crossing the immunological (and mental) barrier. Indian J Nephrol. 2016;26:113-8.
- [Google Scholar]
- Outcome of ABO-incompatible living donor renal transplantations: A single-center experience from Eastern India. Transplant Proc. 2016;48:2622-8.
- [Google Scholar]
- ABO-incompatible living kidney transplants: Evolution of outcomes and immunosuppressive management. Am J Transplant. 2016;16:886-96.
- [Google Scholar]
- ABO incompatible renal transplantation: A paradigm ready for broad implementation. Transplantation. 2009;87:1246-55.
- [Google Scholar]
- Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: Results from 101 centers. Transplantation. 2015;99:400-4.
- [Google Scholar]
- Clinical outcomes after ABO-incompatible renal transplantation: A systematic review and meta-analysis. The Lancet. 2019;393:2059-72.
- [Google Scholar]
- Comparative analysis of ABO-incompatible kidney transplantation with ABO-compatible transplantation: A single-center experience from Eastern India. Saudi J Kidney Dis Transpl. 2019;30:97-107.
- [Google Scholar]
- One hundred ABO-incompatible kidney transplantations between 2004 and 2014: A single-centre experience. Nephrol Dial Transplant. 2016;31:663-71.
- [Google Scholar]
- Current protocols and outcomes of ABO-incompatible kidney transplantation based on a single-center experience. Transl Androl Urol. 2019;8:126-33.
- [Google Scholar]
- Long-term outcomes of ABO-incompatible living donor kidney transplantation: A comparative analysis. Transplant Proc. 2015;47:1720-6.
- [Google Scholar]
- preconditioning therapy in abo-incompatible living kidney transplantation: A systematic review and meta-analysis. Transplantation. 2016;100:933-42.
- [Google Scholar]







