Translate this page into:
Liver biopsy in patients on hemodialysis with hepatitis C virus infection: An important tool
Address for correspondence: Dr. Sanjay Kumar Agarwal, Department of Nephrology, All India Institute of Medical Sciences, New Delhi - 110 029, India. E-mail: skagarwalnephro@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hepatitis C virus (HCV) infection is commonest blood borne infection amongst hemodialysis patients. Still, there is paucity of data on liver biopsy in these patients. Our center is doing regular liver biopsy in these patients and thus thought of sharing our experience. In this retrospective study, all patients with HCV infection on hemodialysis were subjected to liver biopsy. Serum bilirubin, liver enzyme, HCV-PCR, genotype and viral load measurement were done in all. Biopsy specimen was stained with H and E, Periodic Acid Schiff, Gomori Stain, Masson Trichrome and Perls Stain. International Working Group scoring system of Ishak et al. was used for Grading and Staging. Of the 270 liver biopsies, mean age of patients was 34.05 ± 10.28 years and 233 (85.3%) were males. Mean duration of hemodialysis was 10.9 ± 7.4 months while of known HCV infection was 5.2 ± 4.0 months. Genotype 3 was commonest followed by 1. All had normal bilirubin and 64 (23.1%) had normal ALT. In 37 (13.3%) patients anti-HCV was not detectable. Mean histology grade was 4.03 ± 1.65 (1-10) and stage was 0.75 ± 0.98 (0-3). Only one patient had cirrhosis on histology. Associated hemosiderosis was seen 10 patients. Only minor complications were observed with no mortality. In conclusion, our study shows that in one-fourth patients with active liver disease, liver enzymes are persistently normal in patients on hemodialysis. Further, carefully performed liver biopsy is reasonably safe procedure though some patients do have non-fatal complications. Liver biopsy helps in assessing disease activity, which otherwise cannot be assessed. Histological grade and stage in these patients is usually mild and cirrhosis is rare. Till such time other non-invasive test is validated, liver biopsy will remain an important test in these patients.
Keywords
Hemodialysis
hepatitis C virus
liver biopsy
Introduction
Infections are important causes of morbidity and mortality during renal replacement therapy. Hepatitis C virus (HCV) infection is the most common blood-borne infection during hemodialysis (HD) with the prevalence ranging from 6% in the United Kingdom to 60% in Poland and Eastern Europe, and 8–36% in North America. Dialysis Outcomes and Practice Patterns Study survey reported 13.5% mean prevalence of HCV infection in patients on maintenance dialysis from the industrialized world.[1] In India, the prevalence of HCV infection in dialysis patients varies from 2.7% to 45% in other centers.[2345] Since, kidney transplantation confers a definite survival advantage to patients of end stage renal disease (ESRD) even in HCV-infected patients, transplant should be considered the treatment of choice in these patients.[67] Presence of cirrhosis before kidney transplantation is an independent predictor of poor long-term survival.[7] Pretransplant evaluations of HCV-infected kidney transplant candidates require liver biopsy for determination of the severity of liver disease.[8] It has been recommended in kidney disease improving global outcomes guidelines that HCV-infected kidney transplant candidates should undergo a liver biopsy before transplantation, though it has been reported as “weak” evidence. This recommendation is contrary to the American Association for Study of Liver Disease guideline that recommends liver biopsy for patients with genotypes 1 and 4 only, but considers it unnecessary for patients infected with genotypes 2 and 3. The rationale for a liver biopsy in patients on HD is based on the evidences that liver injury markers like liver enzymes do not reliably reflect the histologic severity of disease in this population.[9] Further, 25% of HCV-infected patients evaluated for kidney transplantation have bridging fibrosis or cirrhosis.[9] Also, sequential post-transplant liver biopsies in patients without a pretransplant biopsy have demonstrated that liver histology progresses in about 20% of patients.[10] Liver biopsy before kidney transplantation may also be used as a means to guide antiviral therapy. It is recognized that evaluation of liver injury by noninvasive tests (for example, fibro scan) is an evolving field. The utility of such investigations for assessing liver injury in HCV-infected HD patients is yet not known.
Liver biopsy though essential in the evaluation of patients with liver parenchymal disease, is not without risk and complications. Coagulopathy due to hepatocellular dysfunction and thrombocytopenia due to portal hypertension and hypersplenism are major concerns for an increased bleeding risk in patients with clinically overt liver disease.[11] There is a controversy whether any platelet count level or international normalized ratio (INR) derangement truly separates out those patients with liver disease most likely to bleed after liver biopsy.[12] Even with a normal INR and platelet count, there remains a concern about performing liver biopsy because of platelet dysfunction associated with uremia.[13] A sufficiently large core of tissue is crucial for adequate interpretation. Gauge 16 or larger biopsy needles are recommended, with a minimum length of 2.0–2.5 cm. To ensure reproducibility of liver biopsy interpretation, a number of scoring systems have been devised in an attempt to quantify inflammation and fibrosis. There is limited information about use of liver biopsy in patients with HCV in patients on HD. Our center has been doing liver biopsy in all patients on HD with HCV infection, and being prepared for kidney transplant for the last two decades, we thought of sharing our experience in the present study.
Methods
Study design
Retrospective cohort study.
Setting
Hemodialysis unit at our hospital.
Sampling
Nonprobability consecutive sampling.
Inclusion criteria
All patients of ESRD with HCV infection on maintenance HD and being prepared for renal transplant (RT).
Exclusion criteria
-
Patients not willing to consent for liver biopsy
-
Patients of polycystic kidney disease with associated liver cysts
-
Patients on CAPD program.
All patients of ESRD on maintenance HD and accepted for RT program were included in the study. As per need, patient was subjected to twice/thrice a week HD. At the time of accepting the patient for HD, anti-HCV, HBsAg, aspartate transaminase (AST) and alanine transaminase (ALT) tests were done. Hepatitis B- and C-infected patients were dialysed in isolated room. During maintenance HD, all patients were frequently monitored and managed on their own merit as per our policy.[14] HCV infection was diagnosed by detecting anti-HCV antibodies using 3rd generation ELISA test kit (J Mitra and Co. Ltd., India). Serum bilirubin, AST and alanine transaminase (ALT) tests were done using standard laboratory techniques.
Hepatitis C virus-RNA was determined by real time polymerase chain reaction (PCR) and involved sequence-specific amplification. This was done on real time PCR by using TaqMan method. Dual-labeled fluorogenic probes were optimized for use in the 5'- nuclease (TaqMan) assay. Each test was performed with a positive and negative control. HCV genotyping was determined by PCR and involved sequence-specific amplification. Analysis was based on PCR of the core region with the genotype-specific primers, which allows for the determination of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a and 6a. Each test was performed with positive and negative controls. The PCR products were analyzed using electrophoresis and Gel Doc Systems. For viral load, the amplified product was detected via fluorescent dyes, which were linked to oligonucleotide probes, which bind specifically to the amplified product. Monitoring the fluorescence intensities during the PCR run allows the detection and quantification of the accumulation product, which was monitored on the desktop of a computer. Nucleic acid extraction columns from QIAGEN Hamburg were used; internal controls were added to lysis buffer to monitor and check for PCR inhibition. PCR master mix HCV RG RT-PCR reagents (QIAGEN Hamburg) were used. Total 10 μl of RNA and 15 μl of Master Mix were added to 0.2 ml eppendorf tubes and loaded onto the real time PCR machine (Artus 3000TM).
All patients were subjected to liver biopsy for assessment of their fitness for RT. Patient was dialysed a day prior to liver biopsy without anticoagulation. Prothrombin time and X-ray chest was done prior to biopsy. Surface marking without ultrasound guidance was used while doing liver biopsy. One space below the upper border of liver, point was chosen at mid-axillary line for biopsy. Liver biopsy was done using 18-gauge automatic disposable biopsy gun. A single core of 2–3 cm was taken during biopsy. Patient was monitored for the next 6 h followed by limited mobility for another 12 h. Any complication was assessed and managed on its own merit.
Liver biopsy was fixed in neutral formalin, embedded in paraffin and processed. Sections of liver biopsies, 5 μm in thickness were stained with the following stains routinely in our institution: Hematoxylin and eosin, periodic acid-Schiff after diastase digestion, reticulin stain (Gomori) and Masson trichrome. The Perls stain is performed for visualizing hemosiderin deposition. We follow the International Working Group scoring system of Ishak et al. for grading and staging liver biopsies in chronic hepatitis. In the system of Ishak et al., grading is based on addition of individual scores for the severity of interphase hepatitis/piece-meal necrosis, lobular confluent necrosis (necrosis of groups of hepatocytes), lobular spotty necrosis (single or small focus of necrosis) and portal inflammation. The maximum score for grading is 18 and for staging is 6 (modified Ishak).[15]
Results
Since 1995, 422 liver biopsies have been done in our department of nephrology by the nephrologists. Of these, 92 were in hepatitis B viral infection, 30 were without any known viral infection and 23 were post-transplant liver biopsies. Thus, 277 were in HCV infection before RT. Of these 277, 7 patients had combined HCV and hepatitis B virus infection. These 277 formed the subject for analysis.
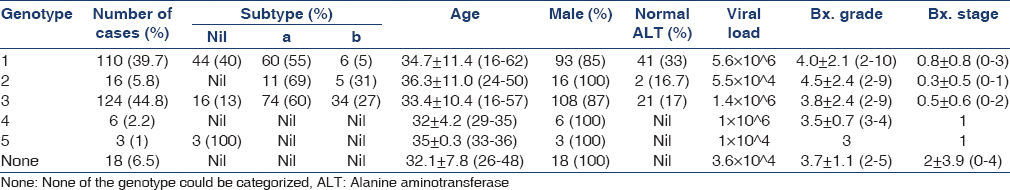
Mean age of patients was 34.05 ± 10.28 (15–67) years and 233 (85.3%) were males. Mean duration of HD before liver biopsy was 10.9 ± 7.4 (2–56) months while mean duration of known HCV infection (with anti-HCV and/or HCV-RNA) before liver biopsy was 5.2 ± 4.0 (1–34) months. Sixty-four (23.1%) patients had ALT values persistently below upper limit of normal of our lab (<50 IU/dl) before liver biopsy. Of the patients who had high ALT values, mean ALT was 118.76 ± 112.5 (51–652) IU/dl. None of the patients in the study group had elevated serum bilirubin level before biopsy at any stage. In view of the type of patients, absence of any symptoms, normal bilirubin, variable transaminases, it was not possible to label and differentiate these patients into acute HCV hepatitis or chronic HCV hepatitis before liver biopsy. None of the patient had clinical signs of liver dysfunction and features of portal hypertension on endoscopy and/or ultrasound abdomen examination. Total 37 (13.3%) patients had high ALT values but normal anti-HCV and the diagnosis of HCV infection was made by detecting HCV-RNA by PCR testing. Mean grade of liver histology was 4.03 ± 1.65 (1–10) and mean stage was 0.75 ± 0.98 (0–3). We further grouped patients with different degrees of grading on histology: Mild (grade 1–3), moderate (grade 4–6) and severe (more than grade 6). Mild necro-inflammation was seen in 124 (45.9%), moderate in 121 (45%) and severe in 25 (9.3%) patients. Similarly, stage 0 on histology was seen in 119 (44.2%), stage 1 in 114 (42.2%), stage 2 in 31 (11.5%) and stage 3 in 5 (1.8%) patients. Only 1 patient had cirrhosis (stage 4) on histology. Associated hemosiderosis was seen 10 patients; mild in 4, moderate in 3 and severe in 3 patients. No patient was documented to have non-HCV-related liver disease. Table 1 shows pattern of genotype seen in the study with relative differences in important variables among different genotype.
In term of complications, 166 (60%) patients did had minor pain; 75 (27%) at local site and 88 (32%) patients had shoulder pain. Forty-five (15.8%) patients required additional analgesia after the biopsy. Thirty-six (13%) patients had some drop in blood pressure and required additional fluid and 13 (4.7%) of them required vassopressors for hemodynamic support. Total 24 (9%) patients required blood transfusion. Two patients required surgical intervention; 1 required stoppage of bleeding at surface of liver and 1 patient required abdominal exploration for removal of pelvic blood collection. None of the patients had mortality related to liver biopsy.
We tried to analyze association of age, sex, genotype, viral load, duration of dialysis (DOD) and duration of known HCV infection with the grade and stage of liver biopsy. Other than age having some correlation (P = 0.04), no other tested variable was having statistically significant association with histology.
Discussion
The clinicopathological features and prognostic indicators of HCV-related liver disease in ESRD patients on maintenance HD have not been well-defined. Not only this, percutaneous liver biopsy is often reported to be contraindicated in dialysis patients due to platelet dysfunction and impaired blood coagulation in uremia.[16] Patients under regular HD are found to have lower ALT/AST levels, lower grading and staging in histology, and lower viral load than nondialyzed individuals with chronic HCV infection.[16] A number of factors have been suggested to explain these findings like immunosuppression, uremia and dialysis itself. Thus, ALT/AST levels are not considered reliable for assessment of hepatic disease activity in these patients. There are limited numbers of studies on liver histology in patients on maintenance HD with HCV infection. After excluding studies that had clubbed dialysis and transplant patients,[171819] we could find 9 studies between 1997 and 2011, which have reported liver histopathology in patients on maintenance HD[192021222324252627] [Table 2]. Of these 9 studies, 5 are from Turkey. None of the studies had mentioned complication rates in their patients, which is one of the major issues while recommending/not recommending liver biopsy in these patients. Thus, we cannot compare complication rate of the procedure in our study with other studies. To decrease the potential complications, we did use thin needle (18G) as compared to conventionally recommended size (16G) needle.
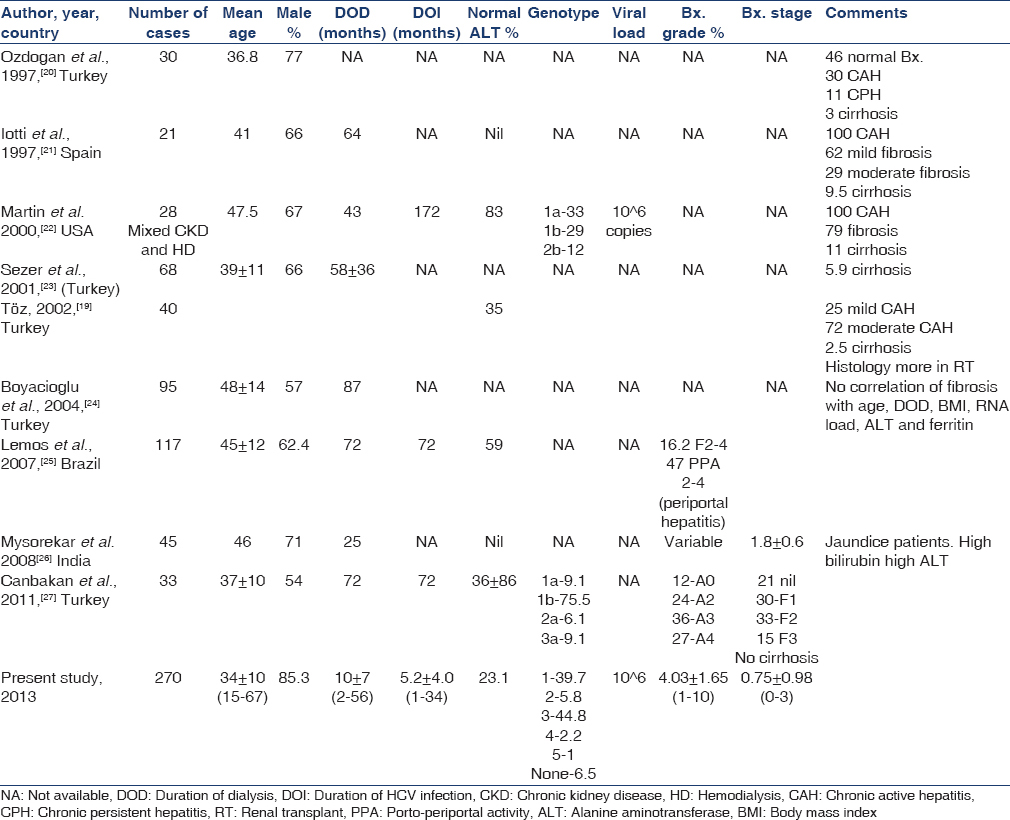
Number of patients included in these studies varied from 21 to 117, while the present study had 270 patients; mean age of patients in previous studies had ranged between third and fourth decade, similar to the mean age of our patients, which is comparable to patients being dialysed and transplanted in our hospitals in the last three decades (unpublished data). We had 85% male patients and this may be because of social factors, as more males are being dialysed in India and more males get RT compared to females. As ours is primarily a unit for RT program, it is not unexpected that we have majority of males in our study. However, other studies also had male preponderance. DOD is likely to have impact on incidence of infection and thus also duration of HCV infection (DOI). DOI is one of the factors affecting the changes on liver histology. Mean DOD at the time of liver biopsy has been reported by most of the studies and has ranged between 25 and 87 months in various studies. However, in our cohort of patients, it was 10 ± 7 (2–56) months. As our center does not do maintenance HD and as most of our patients go for RT with living related donor, a shorter DOD in our set-up is not unexpected. As against DOD, duration of known HCV infection had not been reported by most of the studies. Only 3 previous studies had reported DOI and had been 72[2225] and 172 months.[27] In our set-up, known DOI has been 5.2 ± 4.0 (1–34) months. As the exact time of HCV infection is often difficult to assess due to various reasons, this reported DOI may not be true as time lag from the time of infection to the first diagnosis of HCV infection varies in different patients.
Liver biopsy and simultaneous assessment of liver enzymes is the most reliable to assess the importance of enzymes in these patients in term of disease activity. Five studies have not reported liver enzyme values in relation to biopsy. However, the studies that have reported these values, 35%,[19]59%[25] and 83%[22] patients were reported to have normal liver enzymes in their patients. In our study, persistent normal liver enzymes were seen in 23.1% patients. It overall suggests that a large proportion of these patients will have normal liver enzymes and therefore, liver enzymes are unreliable for assessment of disease activity as well as biochemical remission following treatment. Majority of the studies had not reported genotype of their patients. Only two previous studies have reported genotype[2227] and the commonest genotype was genotype-1, while in our patients the commonest genotype was genotype-3. There is a difference in prevalence of genotypes in different parts of the word and within India. In North India, genotype-3 is the most predominant followed by genotype-1, while in South India it is just the reverse.[28] Though our center is in North India, and we also had genotype-3 being the commonest, this may not be applicable to our patients as such as in our center patients come from all over India and unless their period of stay and other factors are analyzed, geographical factor cannot be attributed to the type of genotype prevalent in our center. There is some suggestion that in genotype-1, viral load and enzyme activity is higher as compared to genotype-3.[28] However, in our cohort of patients, viral load is similar in both genotype-1 and 3 and in fact more patients in genotype-1 had normal liver enzyme as compared to genotype-3 (33% vs. 17%). This suggests that the finding in nondialysis patients in relation to genotype and other markers cannot be automatically translated to dialysis population. Only one previous study had reported viral load in these patients[22] to be 106 copies, which is similar to viral load in our patients.
It is difficult to compare different studies in relation to liver histology. Older studies had reported liver histology in terms of chronic persistent hepatitis, chronic active hepatitis and cirrhosis, and degree of fibrosis in terms of mild, moderate and severe. It is only recent studies,[252627] which had graded the activity and fibrosis. One thing is very clear that in spite of many years of HCV infection at the time of biopsy, cirrhosis have been reported minimally from nil to 11% of patients in different series. In our cohort of 270 cases, only 1 patient showed histological cirrhosis. Except a study by Ozdogan et al.,[20] almost all other studies showed active hepatitis with varying severity in all the patients biopsied. Some of the studies had tried to correlate various variables with the degree of histological changes, however, data is limited. Only a study by Boyacioglu et al.[24] had showed no correlation of histology with age of patient, DOD, body mass index, HCV viral load, serum ferritin and liver enzyme.
Finally, there is an issue that if we have a validated noninvasive test to detect degree of fibrosis, then even current complication rate of liver biopsy will also be avoidable. In this regard, a recent publication[29] while comparing transient elastography (TE) and aspartate aminotransferase-to-platelet ratio index (APRI) with percutaneous liver biopsy in patients of chronic hepatitis on HD, has shown that there was good correlation of TE with histology on biopsy as compared to APRI in predicting patients with significant hepatic fibrosis, advanced hepatic fibrosis and cirrhosis. Liver stiffness measurements of 5.3, 8.3 and 9.2 kPa had high sensitivity (93–100%) and specificity (88–99%) of the patients with a fibrosis stage of F2, F3 and F4, respectively. If these results are validated with other studies in these patients, in future we may not need liver biopsy in these patients.
Conclusion
Our study shows that in one-fourth patients with active liver disease, liver enzymes are persistently normal in patients on HD. Further, carefully performed liver biopsy is a reasonably safe procedure though some patients do have nonfatal complications. Liver biopsy helps in assessing disease activity, which otherwise cannot be assessed. Histological grade and stage in these patients is usually mild and cirrhosis is rare. Till the time any other noninvasive test is validated, liver biopsy will remain an important test in these patients.
Acknowledgment
We would like to thank all the faculty and residents of the department of nephrology helping to do the liver biopsy and collection of data of the patients in the last two decades.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2004;65:2335-42.
- [Google Scholar]
- Prevalence of hepatitis C virus infection in liver disease, renal disease and voluntary blood donors in South India. Indian J Med Microbiol. 1993;11:291-7.
- [Google Scholar]
- Prevalence of anti-HCV antibodies in western India. Indian J Med Res. 1995;101:91-3.
- [Google Scholar]
- Prevalence of anti-HCV antibodies in central India. Indian J Med Res. 1996;104:177-81.
- [Google Scholar]
- Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant. 2005;5:139-44.
- [Google Scholar]
- The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis. 1997;29:608-14.
- [Google Scholar]
- Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257-63.
- [Google Scholar]
- Guideline 4: Management of HCV-infected patients before and after kidney transplantation. Kidney Int. 2008;73(Suppl):109:S53-68.
- [Google Scholar]
- Liver damage in hemodialysis patients with hepatitis C virus viremia: A prospective 10-year study. Dig Dis Sci. 2000;45:2221-8.
- [Google Scholar]
- Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant. 2005;5:1704-12.
- [Google Scholar]
- Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039-46.
- [Google Scholar]
- Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005;45:1413-25.
- [Google Scholar]
- American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-44.
- [Google Scholar]
- Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis. 2005;7:99-108.
- [Google Scholar]
- Viral hepatitis infections in chronic kidney disease patients and renal transplant recipients. Kidney Blood Press Res. 2012;35:454-67.
- [Google Scholar]
- Comparison of clinical features and liver histology in hepatitis C-positive dialysis patients and renal transplant recipients. Am J Gastroenterol. 1999;94:159-63.
- [Google Scholar]
- Long-term impact of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology. 2002;123:1494-9.
- [Google Scholar]
- Clinicopathological features of hepatitis C virus infection in dialysis and renal transplantation. J Nephrol. 2002;15:308-12.
- [Google Scholar]
- Histopathological impacts of hepatitis virus infection in hemodialysis patients: Should liver biopsy be performed before renal transplantation? Artif Organs. 1997;21:355-8.
- [Google Scholar]
- Liver histology in chronic hemodialysis patients infected with hepatitis C virus. Medicina (B Aires). 1997;57:541-5.
- [Google Scholar]
- Histopathological features of hepatitis C in renal transplant candidates. Transplantation. 2000;69:1479-84.
- [Google Scholar]
- Spectrum of liver damage and correlation with clinical and laboratory parameters in HCV infected hemodialysis patients. Ren Fail. 2001;23:807-18.
- [Google Scholar]
- Investigation of possible clinical and laboratory predictors of liver fibrosis in hemodialysis patients infected with hepatitis C virus. Transplant Proc. 2004;36:50-2.
- [Google Scholar]
- Hepatitis C in chronic kidney disease: Predialysis patients present more severe histological liver injury than hemodialysis patients? Am J Nephrol. 2007;27:191-6.
- [Google Scholar]
- Liver histology in patients on hemodialysis with chronic hepatitis C viral infection. Indian J Pathol Microbiol. 2008;51:182-5.
- [Google Scholar]
- Validation of biochemical markers for the prediction of liver fibrosis and necroinflammatory activity in hemodialysis patients with chronic hepatitis C. Nephron Clin Pract. 2011;117:c289-95.
- [Google Scholar]
- Distribution pattern of HCV genotypes and amp; its association with viral load. Indian J Med Res. 2011;133:326-31.
- [Google Scholar]
- Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6:1057-65.
- [Google Scholar]







