Translate this page into:
Long-term Outcome of Lupus Nephritis: A Single Center Study
Address for correspondence: Dr. S. Renuka, Professor of Nephrology, St. Johns Medical College, Sarjapur Road, Opp BDA Complex, Bengaluru - 560 034, Karnataka, India. E-mail: renuka.nephro@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There is paucity of data on long-term outcome of lupus nephritis (LN) from south India. Our study looks at long term outcomes in patients with biopsy proven LN with terms of response to therapy, flare, mortality, treatment-related complications, outcome and post flare analysis. We also analyzed the factors which predicted the outcome.
Methods:
A retrospective observational study was conducted from 2005 to 2012 at St. John's Medical college and hospital, Bangalore. Patients received treatment as per NIH protocol. Patients of LN who regularly visited OPD were included. Statistical analysis was done by using SPSS 19 software.
Results:
At end of 84 ± 6 months (N = 59), 38 subjects showed complete remission (CR), 6 partial remission (PR), 3 developed chronic kidney disease, 2 developed end-stage kidney disease (ESKD), and 10 died. In outcome of flare (N = 15), 7 had nephrotic flare, 3 had refractory flare, 3 showed nephritic flare with 2 developing ESKD. Change of LN class was 3 subjects had changed to class II from class IV, 1 to class II from class V, 7 to class III from class IV, 2 to class IV from class V, and 2 to class IV from class VI. Factors predicting poor outcomes were serum creatinine, hypertension at presentation, and failure to achieve remission in 1 year. 7 subjects conceived, of which 4 of them were treated with azathioprine (AZA) and 3 of them who were on mycophenolate mofetil and was changed to AZA. 4 subjects had successful pregnancy outcome, 2 had preeclampsia, and 1 subject had missed abortion.
Conclusion:
At end of 84 ± 6 months, patient survival rate was 84%.
Keywords
CKD
flare
nephritis
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is defined as a multisystem autoimmune disease. Renal involvement has been the major reason for morbidity and mortality of patients with SLE.[1] Lupus nephritis (LN) has varied presentations ranging from mild asymptomatic proteinuria to rapidly progressive glomerulonephritis.[2] The incidence of kidney involvement differs with ethnicity. Caucasians (European, European Americans; 12-33%) are less likely to have LN than black (African American, Afro-Caribbean; 40-69%), Hispanic (36-61%), or Asian (Indian, Chinese; 47-53%) patients. Most of the data on long-term follow-up available at present are from Western literature. However, no data are available from South India regarding the long-term outcome with treatment; hence, this study was undertaken. Primary aim of our study was to assess the long-term outcomes in patients with biopsy proven LN with terms of response to therapy, flare, mortality, treatment-related complications, outcome post flare. Secondary aim was to analyze the predictors of primary outcome.
Methods
A retrospective study was conducted from 2005 to 2012 at St. John's Medical College and Hospital, Bangalore. Inclusion criteria were patients with age >18 years, regular for follow-up, compliance with medication, and willing to participate in study. Exclusion criteria were age <18 years, patients who lost to follow up and noncompliant with medications and whose record were incomplete. Patients with class VI were excluded. After obtaining ethical committee clearance, clinical details were noted from patient's records, including age of onset, time to onset of nephritis, clinical features along with renal symptoms at onset of kidney disease, and co-morbidities. Routine blood and urine analysis along with anti-nuclear antibodies (ANA), anti- double stranded DNA (anti-DsDNA), and complements (C3, C4) levels were recorded. Renal biopsy was performed for all patients and classified based on International society of nephrology/Renal pathology society ISN/RPS classification. These patients were followed every 3-4 months for 7 years.
Induction as per National Institute of Health (NIH) protocol and maintenance therapy using Azathioprin/Mycophenolate mofetil (MMF). NIH protocol consisted of intravenous pulse cyclophosphamide (0.5-1 gm/m2) monthly for 6 months adjusted to white blood cell nadir, given monthly for the first six months then quarterly for at least 12 months.[3] Patients with proliferative forms of LN were treated with oral corticosteroids, typically prednisone starting at 1 mg/kg/day and tapered over weeks to months. In severe disease with rapid deterioration of kidney function, high-dose intravenous methylprednisolone (10-15 mg/Kg BW/day × 3 days) was administered followed by oral corticosteroids 1 mg/kg BW. The dose of MMF used was 1.5 gm/d and AZA used was 2-2.5 mg/kg/day. The maximum dose of hydroxychloroquine used was 6-6.5 mg per kilo body weight. The dose of angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) was titrated to maximum tolerated doses with periodic monitoring of blood urea, serum creatinine, and serum potassium. The target blood pressure was 120/80 mm of Hg. Once complete remission was achieved, maintenance therapy was continued for at least 1 year before the tapering of immunosuppression. The relapse of LN after complete or partial remission was treated with the initial therapy followed by the maintenance therapy that was effective in inducing the original remission. The average duration of immunosuppression was 4.5 ± 1 years.
Outcome definitions used were; complete renal remission (CR) – urine proteinuria <150 mg/dl, normal renal functions, and normal serum albumin, partial remission (PR) – urine proteinuria >150 mg-2000 mg with normal serum creatinine, nephritic flare – increase in serum creatinine by 30% with active urine sediment and proteinuria flare – increase in proteinuria by ≥30%, treatment failure – failure to reduce proteinuria by 50% or a sustained increase in serum creatinine by 30%.
Statistical analysis was done by using SPSS 19 software. Continuous variables were analyzed using Student's t-test. All data are presented as means ± standard deviation (SD) Pearson's correlation test used for correlating variables. Kaplan–Meier curve used for calculating survival. P value <0.05 was considered as statistically significant.
Results
A total of 71 patients were included, 4 had dropped out of study, another 4 had stopped the medications and 2 patients lost for follow up. 2 patients with class VI lupus nephritis were excluded. A total of 59 patients were included of which females were predominant (83.3%). Mean duration of follow-up was 84 ± 6 months and average age of the study group was 32 ± 6 years. Mean duration of disease before including into the study was 14 ± 4 months [Table 1]. Distribution of class of LN and intial presentation is depicted in Table 1. 52 patients had hematological abnormalities which included anemia, thrombocytopenia and leucopenia. At the onset, 27 subjects had proteinuria (>3.5 gm/1.73 m2 body surface area) and 9 had serum creatinine >1.5 mg/dl. The class of LN were as follows at the time of renal biopsy: Class II (10), Class III (7), Class IV (39), and Class V (4). A total of 26 subjects had nephritic syndrome, 12 had subnephritic proteinuria, 17 had nephrotic syndrome, 4 patients had RPGN, and 2 developed CKD in the study.
| Baseline characteristics | Clinical features at onset (n=number of patients) |
|---|---|
| Age | 32±6 years |
| Sex (female) | 51 |
| Fever | 38 |
| Proteinuria (>3.5 gm/1.73 m2) | 27 |
| Oral ulcer | 27 |
| S. creatinine >1.5 mg/dl | 9 |
| Alopecia | 19 |
| ANA positive | 58 |
| Photosensitivity | 17 |
| Anti-DsDNA positive | 33 |
| Arthritis | 45 |
| Low complements (C3, C4) | 50 |
| Hematological abnormalities | 52 |
| Class II LN | 10 |
| Class III LN | 7 |
| Class IV LN | 39 |
| Class V LN | 4 |
Primary outcome
At the end of 84 ± 6 months (N = 59), 38 patients had complete remission, 6 patients had partial remission, 3 patients developed CKD, 2 patients developed ESKD, and 10 patients expired [Figure 1]. Cyclophosphamide (CYP)/AZA has its significant effect on long-term renal survival in subjects with complete CR (24%) and partial or remission PR (11%). CYP/MMF had showed CR in 13% and PR in 8%. CYP/CYP and Cyclosporine had showed CR in 2% and 1% respectively. In the subjects receiving MMF, 6 developed leucopenia and 7 intractable diarrhoea. Whereas, 13 subjects receiving azathioprine developed leucopenia [Figure 2]. In outcomes of flare (N = 15), 7 had nephrotic flare, 3 had refractory flare, 3 developednephritic flare, and 2 developed ESRD. Out come of flare is shown in [Figure 3]. 59 patients were included for survival analysis. At the end of 84 months, survival rate was 84% (n = 49) and 16% (n = 10) died [Figure 4]. Factors predicting poor outcomes were serum creatinine and hypertension at onset, and failure to achieve remission in 1 year.

- Flow chart showing response to treatment
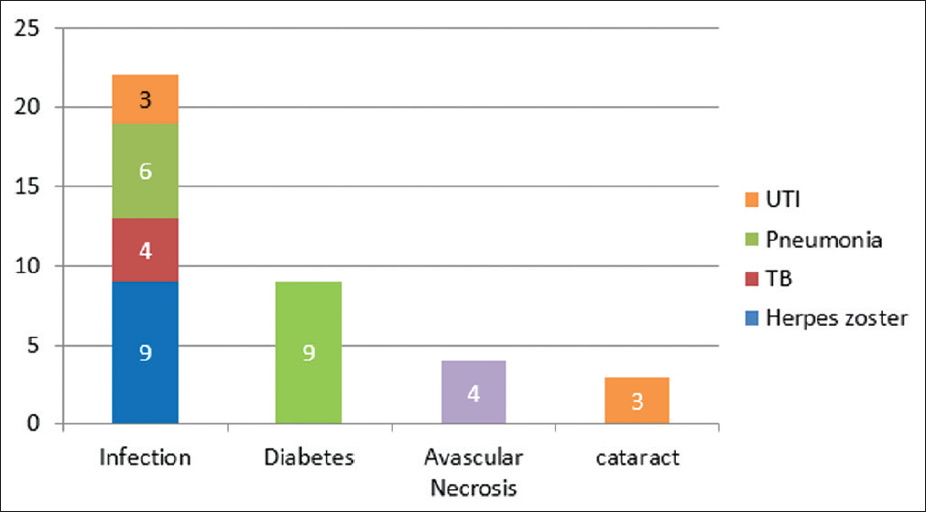
- Treatment-related complications
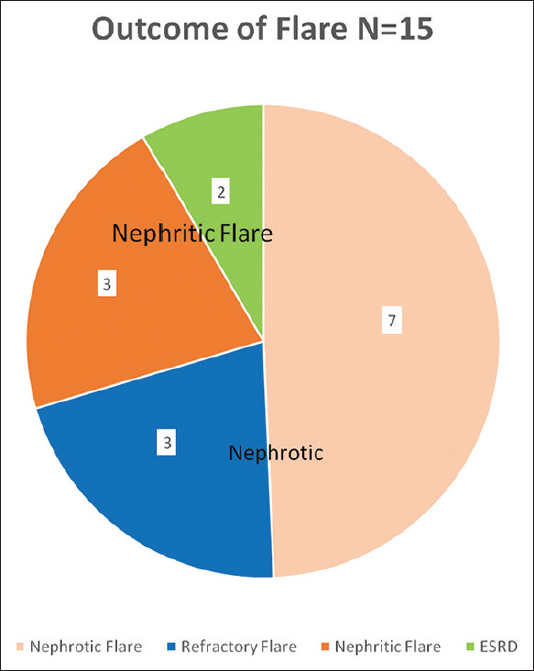
- Outcome of flares
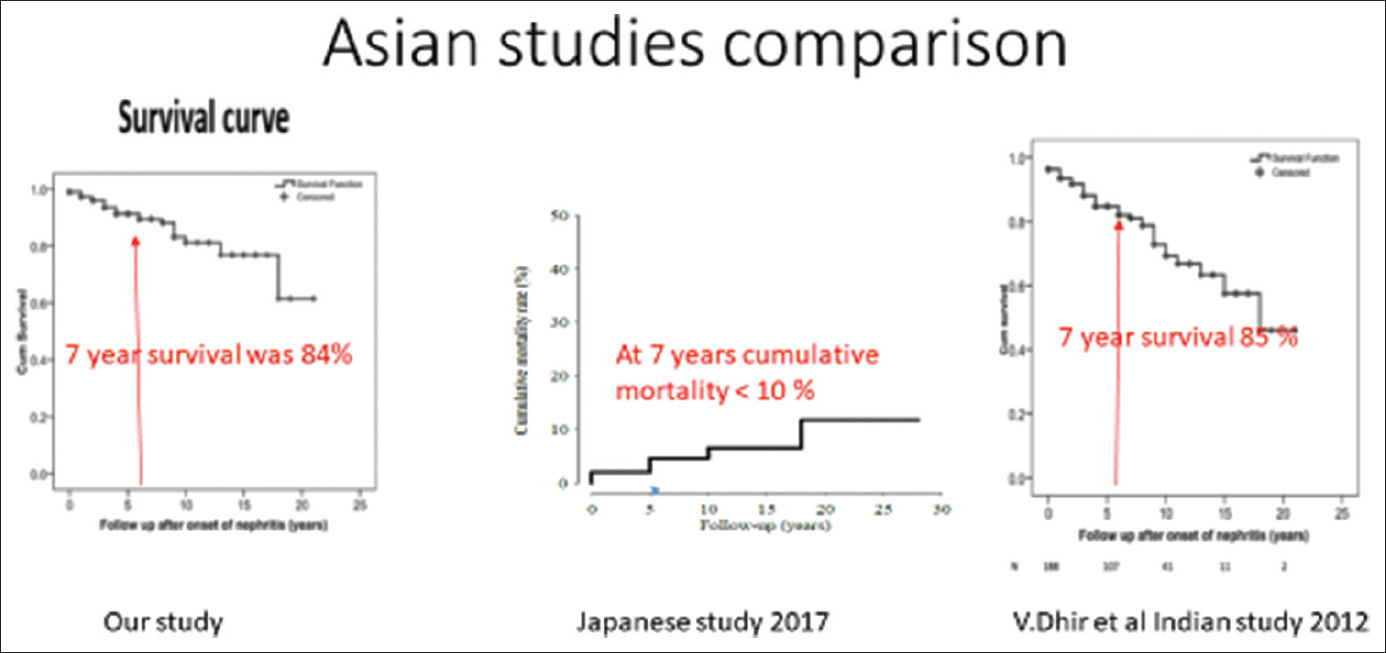
- Showing survival analysis comparing with other studies
In relation to change of LN class, 3 subjects had changed to Class IV from Class II,1 Class V from Class II, 7 Class IV from Class III, 2 Class IV from Class V . In our study population during follow-up, seven subjects conceived, 4 of them were treated with AZA and 3 of them were on MMF (changed to AZA); 4 subject had successful pregnancy outcomes, 2 developed preeclampsia, and 1 subject had missed abortion. The symptoms (alopecia, arthritis, oral ulcers) completely disappeared in the patients. Also there was normalisation of hematological abnormalities in the patients by the end of 6 months.
Discussion
Renal involvement in SLE is one of the most serious manifestations. Therapy of LN, especially proliferative forms, requires prolonged aggressive therapy, with aim to reduce disease progression and improve long-term outcome of patient with LN. “NIH protocol” (The National Institutes of Health) of intravenous pulsed CYP had been the standard for treatment of LN.[4] Cyclophosphamide has been shown to be a potent immunosuppressive agent which leads to a good control of the disease. It has improved the prognosis of LN over the years. Cameron et al. found that life expectancy at 5 years increased from 44% in the period 1953-1969 to 82% in the period 1980-1995.[5] In a Italian study, which recruited 93 patients who were followed for a median of 15 years, renal survival was 97% at 10 years and 82% at 20 years.[6] Data presented in this study of 61 patient , the records were traced upto 7 years. This is first of its kind of study from south India. As this was a retrospective study there were a few difficulties in gathering all records. In our study, Class IV was the most common histology and 43.3% patient had nephrotic range proteinuria. This findings was similar as in other studies.[123456] 38 patient who showed CR were treated with CYP/AZA and had significant effect on long term survival. Similar results were seen by Senija Rašić et al.[1] A total of 15 patients developed flare and most common was nephrotic flare (7 patients). Whenever there was flare, a repeat renal biopsy was performed and class transformation was seen. Most frequent class transformation was from Class III to Class IV . In cases of flare , usefulness of repeat renal biopsy is controversial.[7] Current evidences suggest histological transformation is common in nonproliferative lesions. In great majority of studies, Class II progressed to higher grade nephritis, resulting in worse prognosis. Most of these patients progressed to a proliferative Class III or IV and less freqently Class V. The frequency of pathological conversion of Class V to proliferative class is less likely. Therefore non-proliferative Class II or V may benefit with repeat biopsy. In our study, proliferative group biopsied remained proliferative in repeat biopsy and Class II progressed to Class IV and Class V. Two patient progessed from Classes IV to VI.[8910]
Steroids plays an important role in the therapy of SLE, but their management also associated with a whole series of adverse effects, especially those that were administrated in the long term, but can occur in the short term. At the metabolic level, glucocorticoids increased the risk of hyperglycemia in both diabetes mellitus (DM) and non-DM patients.[1] Apart from this, cyclophosphamide causes leucopenia, hemmorrahagic cystitis and gonadal toxicites. MMF can cause leucopenia and diarrohea as common complications . Most common immunosuppression-related complications were leucopenia followed by diarrhea.[11]
Renal involvement in the form of either active LN at the time of conception or a LN new onset or flare during pregnancy increases the risks of preterm delivery, pre-eclampsia, maternal mortality, fetal/neonatal demise, and intrauterine growth restriction. Consequently, current recommendations advise that the affected woman achieve a stable remission of her renal disease for at least 6 months before conception.[12] In our study, seven patient conceived out which three were planned pregnancy and their outcome was good. Two unplanned pregnancy developed pre-eclampsia. Those patients who were on MMF and conceived were shifted to Aza.
Previous study in Asian population shown survival rate at the end of 7 years as 85%.[2] Western population shown survival rate at the end of 7 years as 80-85%.[13] According to John Hopkin's study survival rate at the end of 7 years is 98%.[8] Long-term survival reported in Western and Asian countries are same. In our study, mean survival at end of 84 months was 84%. The factors influencing prognosis in LN are variable in literature. In a study by Kammoun et al., the presence of hypertension, renal failure, massive proteinuria, and high activity index score of LN was associated with poor renal prognosis.[14] The predictors of poor outcome in our study were serum creatinine at onset >1.8 mg/dl, hypertension at onset, Class IV LN, and failure to achieve remission in one year. Limitations of the study are single centre and retrospective nature of the study.
Conclusion
Our study shows long-term outcome of LN is in par with other Asain and Western population.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank all patients who gave consent to go through their records and also colleagues from record section , pathology , biostatics, and secretaries who helped in gathering information, analysis, and typing.
References
- Long-term outcome of lupus nephritis in Asian Indians. Arthritis Care Res (Hoboken). 2012;64:713-20.
- [Google Scholar]
- Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614-9.
- [Google Scholar]
- NIH conference. Systemic lupus erythematosus: Evolving concepts. Ann Intern Med. 1979;91:587-604.
- [Google Scholar]
- The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant. 2007;22:2531-9.
- [Google Scholar]
- The clinical relevance of repeat biopsy in lupus nephritis flare. Nephrol Dial Transplant. 2009;24:3712-7.
- [Google Scholar]
- Renal remission status and long-term renal survival in patients with lupus nephritis: A retrospective cohort analysis. J Rheumatol. 2018;45:671-7.
- [Google Scholar]
- Lupus nephritis When and How often to do biopsy & what does it mean. J Autoimmune. 2016;74:27-40.
- [Google Scholar]
- Therapy side effects in systemic lupus erythromatosis. Current Health Sci J 2018:44.
- [Google Scholar]
- Obstetric nephrology: Lupus & lupus nephritis in pregnancy. Clin J Am Soc Nephrol. 2012;7:2089-99.
- [Google Scholar]
- Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken). 2010;62:873-80.
- [Google Scholar]
- Poor prognostic factors of lupus nephritis. Saudi J Kidney Transpl. 2011;22:727-32.
- [Google Scholar]







