Translate this page into:
Long-Term Outcomes of Anticomplement Factor H Antibody Positive Versus Negative Atypical Hemolytic Uremic Syndrome
*Vamsidhar Veeranki and Jeyakumar Meyyappan contributed equally and should be considered for the first joint authorship.
Corresponding author: Narayan Prasad, Department of Nephrology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India. E-mail: narayan.nephro@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Veeranki V, Meyyappan J, Srivastava A, Kushwaha RS, Behera M, Patel MR, et al. Long-Term Outcomes of Anticomplement Factor H Antibody Positive Versus Negative Atypical Hemolytic Uremic Syndrome. Indian J Nephrol. 2025;35:402-9. doi: 10.25259/IJN_106_2024
Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is a severe thrombotic microangiopathy predominantly affecting the kidneys, often associated with complement dysregulation. This study is aimed to analyze the clinical characteristics, treatment outcomes, and long-term implications of aHUS in a resource-limited setting.
Materials and Methods
A retrospective observational study conducted at an institute between January 2016 and December 2022 included all patients with aHUS, excluding secondary causes and renal transplant recipients. Demographic profiles, clinical features, laboratory parameters, treatment modalities (immunosuppression and plasma exchange), and outcomes were collected. Anticomplement Factor H (anti-CFH) antibody, complement levels, and genetic mutation analysis were performed to ascertain etiological factors. The patient and renal outcomes of anti-CFH positive and negative patients on long-term follow-up were compared.
Results
Fifty-seven patients (mean age: 12.5 ± 4.9 years; 63% males) were analyzed. Among them, 33 (57.9%) tested positive for anti-CFH antibodies and eight presented postpartum. Initial remission was achieved in 42 (73.6%) patients, with 13 (22.8%) partial and 29 (50.9%) complete remission. The median follow-up duration was 24 months [interquartile range (IQR) 8.5–84]; 12 (21%) patients died, with two deaths during the index admission, six among nonresponders, and 4 among responders. Dialysis-free renal survival was superior in anti-CFH seropositive patients (81.2%) compared to seronegative counterparts (55.9%), while patient survival was statistically similar between the two groups. Elevated anti-CFH titers (>4000 AU/ml), age ≥16 years, female gender, and seizures predicted nonresponsiveness.
Conclusion
Anti-CFH antibody associated aHUS had better kidney outcomes than the seronegative counterparts. In resource limited settings, a combination of plasma exchange and immunosuppression showed promising results in the short and long term.
Keywords
Anti-CFH antibody
Atypical HUS
Long-term outcomes
Plasmapheresis
Thrombotic microangiopathy

Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare but severe form of thrombotic microangiopathy (TMA) that affects multiple organs, the kidneys in particular. It occurs as a consequence of genetic or acquired dysregulation of complement activation, leading to endothelial cell dysfunction and thrombosis of the small vessels, hemolysis, thrombocytopenia, and acute kidney injury (AKI). The incidence ranges between 0.23 and 1.9 per million population annually and a prevalence of two to ten per million population, which varies according to the region and age.1 It may be sporadic or familial and is mostly caused by dysregulation of the alternative complement pathway. The initial attack of aHUS can occur at any age and is associated with a high rate of progression to end-stage kidney disease (ESKD). Approximately 25% patients die during acute phase and 50% progress to ESKD. Many aHUS patients relapse in the native or transplanted kidneys and require close monitoring and long-term management.2–4
Anticomplement therapy, Eculizumab, revolutionized the management of aHUS. However, the availability and cost remained major concerns, especially for people living in low- and middle-income countries.5–11 There are multiple challenges in the management of this disease in resource-limited settings. Lack of a complete diagnostic panel, delayed and late referral to specialized centers, high burden of infection following conventional immunosuppression, and lack of availability of complement inhibitors amplify the burden. A separate guideline for a developing country and in a resource-limited setting has been advocated.12 Outcomes of this disease are likely to vary based on the available diagnostic and therapeutic facilities in a single center. Conventional immunosuppression and plasmapheresis are widely available in tertiary centers in India, and there is a paucity of published literature on renal and patient outcomes of aHUS based on protocols using these modalities alone.
Studies from Indian population revealed more than 50% of aHUS occurs because of anti-factor H antibodies, and plasmapheresis with immunosuppressive therapy may improve the outcomes of the patients.13,14 The use of immunosuppression was heterogeneous, and the long-term outcome data of the treatment are not available. In this study, we analyzed the clinical manifestations, histological findings, and short- and long-term outcomes of aHUS at our institution.
Materials and Methods
The study was designed as a retrospective observational study, which included all patients of aHUS admitted between January 2016 and December 2022, at the institute. The demographic profile, clinical characteristics, biochemical and serological parameters, kidney histological findings, treatment given, and outcomes were retrieved and analyzed. Patients with thrombotic thrombocytopenic purpura (TTP), typical HUS, renal transplant recipients, and patients with secondary causes of HUS were excluded. Anti-CFH antibodies associated aHUS being the commonest in the region, initial diagnostic evaluation involved the assessment of anti-CFH antibodies, complement-3 and 4 levels, and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) assay for patients. Subsequent genetic mutation analysis was pursued if the results of these tests were negative. The institute’s ethics committee has approved the study. The waiver for patient consent was obtained from ethics committee in view of retrospective study.
Definitions and criteria used for diagnosis
HUS was defined as microangiopathic hemolytic anemia and thrombocytopenia (MAHAT) with renal failure.15 AKI in pediatric patients was defined as serum creatinine levels at least 1.5 times the upper limit of the age- and sex-specific pediatric reference range as per KDIGO 2012 guidelines.16 Hypertension in children was defined “as systolic and/or diastolic BP that is ≥95th percentile for age, gender, and height”17 and in adults as “systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg.”18
Management protocol
After confirmation of the diagnosis of aHUS based on clinical and laboratory parameters, the serum sample for anti-CFH antibody titre and ADAMTS13 was collected. Patients were initiated on immunosuppression and plasmapheresis as per our protocol. The initial immunosuppression included intravenous methylprednisolone (10mg/kg/day) for three days, followed by oral prednisolone 1mg/kg/day for two weeks, followed by tapering of 25% of the initial dose every two weeks, and to continue on 5–10 mg per day as maintenance dose. As Eculizumab is not available in India, the addition of steroid-sparing agent mycophenolate mofetil (1000–1200 mg/m2/day) or oral cyclophosphamide (2–2.5 mg/kg/day for three months) was used based on the anti-CFH antibody titre. Genetic analysis of complement dysregulation was done if the patients were willing and affordable.
Plasma exchange was performed using a membrane separation technique through a secured, nontunneled jugular hemodialysis catheter. Plasmapheresis was performed at a rate of 40–60 ml/kg/session, followed by replacement with normal plasma. A minimum of five plasmapheresis sessions were performed in all cases, and further sessions were extended depending on the clinical response. Plasmapheresis was deferred if the patient showed spontaneous renal or hematological remission.
Kidney biopsy was performed in all patients, if there was no thrombocytopenia and coagulopathies. The treatment was initiated after confirmation of diagnosis by noninvasive tests, and biopsy was performed once the thrombocytopenia improved. All patients who were nonresponsive to the initial treatment were subjected to kidney biopsy. Decision to biopsy was at the treating clinician’s discretion.
The outcomes of the patients were analyzed in terms of hematological and renal remission. The immediate 1-month, 6-month, 12-month, and end of the follow-up outcomes were analyzed. We also analyzed the readmissions, relapses, dialysis-free renal survival, patient survival, infections, and other major adverse effects following immunosuppression. The clinical characteristics were compared between the responders (both renal and hematological remission) and nonresponders. The following remission criteria were followed:4
Complete Renal Remission (CRR): Complete normalization of kidney function, including resolution of AKI markers such as serum creatinine. Restoration of normal urine output and reduction or absence of proteinuria.
Partial Renal Remission (PRR): Significant improvement in kidney function with an estimated glomerular filtration rate (eGFR) >30 ml/min/1.73 m2 and related markers, but not achieving complete normalization. Partial resolution of AKI, with a notable reduction in serum creatinine or partial improvement in urine output and proteinuria.
Hematological remission was defined as “Complete recovery of hemolytic anemia and thrombocytopenia.”
Statistical analysis
Statistical analysis was performed using the IBM, SPSS software, version 25. All data were expressed in mean or median, depending on the normality of the data. The Chi-square test or Fischer’s exact test was used to compare the categorical values between the groups as per the application required. Student’s t-test was used to compare the mean values of continuous variables if it was normally distributed or else Mann-Whitney’s U-test was used. Time-dependent multivariate Cox-regression analysis was used to identify factors predicting the responder using ESKD as the event. Death was considered as an event in the case of patient survival analysis, and the permanent dialysis dependency was considered as an event for dialysis-free survival analysis.
Results
The clinical characteristics of the cohort are shown in Table 1. During the study period (2016–2022), 57 consecutive patients with aHUS were included [Figure 1]. The mean age was 12.5 ± 4.9 years, and males were 36 (63.1%). Most patients (73.6%) experienced prodromal symptoms, mainly fever (42.1%) and acute gastroenteritis (15.7%). Oliguria typically occurred seven days (IQR: 3–15) after prodrome onset. Eight patients presented with aHUS in the postpartum period. All patients had hypertension at presentation and oligo-anuria was observed in 47 (82.4%) at presentation. Seizures either at presentation or during the disease were seen in 21 patients (36.8%). Three patients had plasmodium vivax infection and one patient had acute pancreatitis. The patients were followed up for a median of 24 months (IQR: 8.5–84 months).
| Variable | N = 57 |
|---|---|
| Age | 12.5 ± 4.9 |
| Gender, Male (%) | 36 (63.1%) |
| Systolic BP | 137 ± 24.1 |
| Diastolic BP | 86.6 ± 14.1 |
| Index event to HUS; Days, Median (IQR) | 7 (3–15) |
| Duration of oliguria; Days, Median (IQR) | 19 (10–30) |
| No. of packed cell transfusion, Median (IQR) | 3 (2–4) |
| Triggering event | |
| Fever | 24 (42.1%) |
| Acute gastroenteritis | 9 (15.7%) |
| Postpartum | 8 (14%) |
| Malaria (Plasmodium vivax) | 3 (5.2%) |
| Pancreatitis | 1 (1.7%) |
| None | 15 (26.3%) |
| Clinical features at presentation | |
| Oligo-anuria | 47 (82.4%) |
| Seizures | 21 (36.8%) |
| Jaundice | 15 (26.3%) |
| Microscopic haematuria | 47 (82.5%) |
| Macroscopic haematuria | 7 (12.2%) |
| Rash | 6 (10.5%) |
| Laboratory parameters | |
| Hb (mg/dl) | 5.8 ± 1.4 |
| TLC (/μl) | 10861.4 ± 3984.9 |
| Reticulocyte (%) | 8.6 ± 5.8 |
| Schistocytes (%; Median, IQR) | 5.5 (2–10) |
| Platelet count (/μl) | 65709 ± 50801 |
| LDH (IU/L) | 3499.2 ± 2116.4 |
| C3 level (mg/dl) | 75.7 ± 26.5 |
| C4 level (mg/dl) | 23.2 ± 9.43 |
| S. Creatinine (mg/dl) | 7.2 ± 2.6 |
| Low C3 (%) | 13 (22.8%) |
| Transaminitis (%) | 44 (77.2%) |
| Anti-CFH antibody (%) | 33 (57.9%) |
| Duration of follow up in months; Median (IQR) | 24 (8.5–84) |
BP: Blood pressure, HUS: Hemolytic uremic syndrome, Oliguria: Urine output < 100 ml/day, Oligo-anuria: Urine output < 400 ml/day (oliguria) and < 100 ml/day (anuria), IQR: Interquartile range, Hb: Hemoglobin, TLC: Total leukocyte count, LDH: Lactate dehydrogenase, Transaminitis: Elevation of Aspartate aminotransferase and/or alanine aminotransferase by two times upper limit normal for the age; Anti-CFH: Anticomplement factor H.
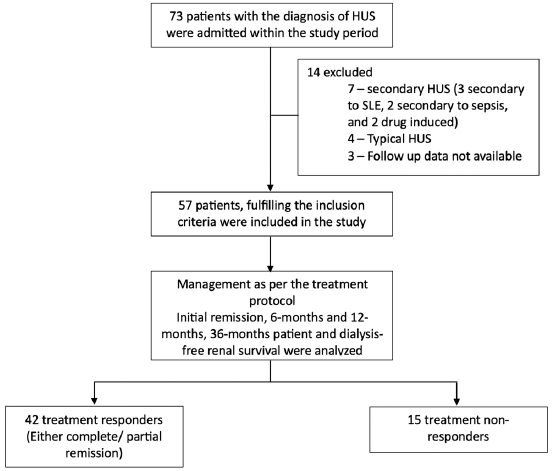
- Flow chart of the study. HUS: Hemolytic uremic syndrome, SLE: Systemic lupus erythematosus.
Serological testing
Anti-CFH positivity was noted in 33 (57.9%) patients. Out of eight patients with postpartum aHUS, two had mutation in the gene encoding membrane cofactor protein (MCP), two had variants of complement factor-B (CFB), and one had complement factor I (CFI) variants. Three patients had complement three related mutation [complement factor H related (CFHR1), CFHR3, and combined CFHR1 and 5]. Out of three patients who were Plasmodium vivax positive (5.2%), two had anti-CFH antibody and one had genetic mutation (CFHR1), indicating Plasmodium vivax infection just as a triggering factor. Thirteen patients had low C3 level, indicating inherent defect in complement 3 dysregulation. Two patients had MCP mutation. One patient of the aHUS with anti-CHF antibody positive patients had acute pancreatitis at presentation.
Responder and nonresponders
Patients were classified into responders and nonresponders based on whether they achieved complete remission (CR)/partial remission (PR) after treatment. The comparison of the baseline characteristics among responders and nonresponders is shown in Table 2. The mean age of nonresponders was significantly higher (18.1 ± 4.3 versus 10.5 ± 3.3 years, p = 0.001) compared to the responders. The proportion of males was significantly higher among nonresponders compared to responders (73.8% versus 33.3%, p = 0.005). Among the clinical and laboratory features, patients with seizures were significantly higher among the nonresponders (42.9% versus 28.6%, p = 0.03) as compared to responders. Thrombocytopenia was more severe (59,431 ± 38,407/μl versus 83,288 ± 74,525/μl) and transaminitis (85.7% versus 53.3%) was more commonly noted in responders compared to the nonresponders. Serum creatinine at presentation was significantly higher among the nonresponders (9.4 ± 1.9 versus 7.2 ± 2.6 mg/dL, p < 0.001). The dialysis requiring AKI was higher in nonresponder (83.3% in responders versus 100% in nonresponders, p = 0.09), although statistically not significant. Anti-CFH positivity was higher among the responders (66.7% versus 33.3%, p = 0.02); within the seropositive subgroup, anti-CFH titres were significantly higher among the nonresponders compared to the responders (7557.6 ± 5277.1 versus 3455.2 ± 1921.3 AU/ml). The details of the histological comparison between responder and nonresponder patients are shown in Table 3.
| Variable | Responders (n = 42) | Nonresponders (n = 15) | p-value |
|---|---|---|---|
| Age | 10.5 ± 3.3 | 18.1 ± 4.3 | 0.001 |
| Gender | 0.005 | ||
| Male (%) | 31 (73.8%) | 5 (33.3%) | |
| Systolic BP | 131 ± 19.4 | 154 ± 28.3 | 0.001 |
| Diastolic BP | 83.3 ± 12.2 | 95.6 ± 15.6 | 0.003 |
| Clinical features at presentation | |||
| Rash | 5 (11.9%) | 1 (6.6%) | 0.56 |
| Oligo-anuria | 34 (80.9%) | 13 (86.6%) | 0.73 |
| Seizures | 12 (28.6%) | 9 (42.9%) | 0.03 |
| Jaundice | 11 (26.2%) | 4 (26.7%) | 0.97 |
| Laboratory parameters | |||
| Hb (gm/dl) | 5.6 ± 1.5 | 6.2 ± 1.1 | 0.16 |
| TLC (/μl) | 10885.7 ± 3854.7 | 10793.3 ± 4471.5 | 0.59 |
| Reticulocyte (%) | 9.3 ± 6.2 | 6.6 ± 3.9 | 0.12 |
| Schistocytes (%; Median, IQR) | 5 (2–10) | 6 (2–12) | 0.18 |
| Platelet count (/μl) | 59431 ± 38407 | 83288 ± 74525 | 0.04 |
| LDH (IU/L) | 3791 ± 2345.5 | 2680 ± 909.6 | 0.08 |
| C3 level (mg/dl) | 73.3 ± 23.7 | 82.2 ± 33.3 | 0.27 |
| C4 level (mg/dl) | 22.2 ± 8.4 | 25.4 ± 11.8 | 0.29 |
| Serum creatinine (mg/dl) | 6.4 ± 2.3 | 9.4 ± 1.9 | <0.001 |
| Low C3 (n, %) | 10 (23.8%) | 3 (20%) | 0.76 |
| Transaminitis (n, %) | 36 (85.7%) | 8 (53.3%) | 0.01 |
| Anti-CFH positive (%) | 28 (66.7%) | 5 (33.3%) | 0.02 |
| Anti-CFH titre (AU/ml) | 3455.2 ± 1921.3 | 7557.6 ± 5277.1 | 0.003 |
| Dialysis requiring renal failure | 35 (83.3%) | 15 (100%) | 0.09 |
| Treatment regimens | |||
| Plasmapheresis | 39 (92.9%) | 12 (80%) | 0.16 |
| Steroids | 31 (73.8%) | 9 (60%) | 0.25 |
| Mycophenolate mofetil | 18 (43.9%) | 6 (40%) | 0.55 |
| Cyclophosphamide | 5 (12.2%) | 1 (6.7%) | 0.55 |
| Number of sessions of plasmapheresis received; Median (IQR) | 6.5 (5–10) | 7 (5.2–11.25) | 0.43 |
| Duration from onset of disease till initiation of plasmapheresis; Median (IQR) | 15 (10–30) | 27.5 (15.7–45) | 0.01 |
BP: Blood pressure, HUS: Hemolytic uremic syndrome, Oliguria: Urine output < 100 ml/day, Oligo-anuria: Urine output < 400 ml/day (oliguria) and < 100 ml/day (anuria), IQR: Interquartile range, Hb: Hemoglobin, TLC: Total leukocyte count, LDH: Lactate Dehydrogenase, Transaminitis: Elevation of Aspartate aminotransferase and/or alanine aminotransferase by two times upper limit normal for the age, Anti-CFH: Anticomplement factor H.
| Biopsy parameter | Responders (n = 42) | Nonresponder (n = 15) | p-value |
|---|---|---|---|
| Mesangiolysis | 20 (47.6%) | 4 (33.3%) | 0.46 |
| Endotheliosis | 19 (45.2%) | 4 (33.3%) | 0.46 |
| Fibrin thrombi | 29 (69%) | 7 (58.3%) | 0.55 |
| Duplication of GBM | 24 (58.8%) | 6 (50%) | 0.63 |
| Ischemic changes in glomeruli (wrinkling of GBM/intima) | 24 (58.8%) | 6 (37.5%) | 0.64 |
| Acute cortical necrosis | 7 (16.7%) | 4 (33.3%) | 0.43 |
| Diffuse global glomerulosclerosis | 3 (7.1%) | 1 (8.3%) | 0.79 |
| IFTA | |||
| Mild (<25%) | 39 (92.8%) | 7 (58.3%) | 0.019 |
| Moderate (25–50%) | 3 (7.2%) | 5 (41.7%) |
GBM: Glomerular basement membrane, IFTA: Interstitial fibrosis and tubular atrophy.
The treatment regimens used among the responders and nonresponders were noted to be similar. The median duration of initiation of plasma exchange from the onset of disease was significantly higher among the nonresponders compared to the responders (27.5 days, IQR: 15.7–45 versus 15 days, IQR: 10–30, respectively, p = 0.01). Among the responders, the median time to initial response in terms of improvement in hematological remission was 15 days (IQR: 10–23 days). The median time to renal remission was 43 days (18–64 days) and was 28 (IQR: 9–37 days) in anti-CFH positive versus 52 days (IQR: 23–72) in anti-CFH negative group (p = 0.03).
On time-dependent multivariate Cox-regression analysis [Table 4], we observed that age ≥16 years, female gender, development of seizure, anti-CHF titre > 4000 were significantly associated with nonresponsiveness. Severe hypertension and transaminitis were not associated with responsiveness.
| Variable | Odds ratio | 95% CI | P values |
|---|---|---|---|
| Age ≥16 years | 20.5 | 7.8–468.3 | <0.001 |
| Gender: Female | 5.5 | 1.16–26.07 | 0.02 |
| Seizures | 5.8 | 1.1–34.7 | <0.001 |
| Accelerated hypertension | 8.1 | 0.42–15.4 | 0.16 |
| Transaminitis | 0.28 | 0.04–1.69 | 0.53 |
| Anti-CFH positivity | 4.3 | 0.68–27.4 | 0.10 |
| Anti-CFH titre ≥4000 | 18.6 | 5.6–333.8 | <0.001 |
Anti-CFH: Anticomplement factor H antibody, CI: Confidence intervals
Long-term outcomes
Patient survival
The overall mortality among the cohort was 12 (21%). During the index admission, two patients succumbed to intracranial bleeding soon after admission and ten patients—six nonresponders and four responders with PR—died during the follow-up period. Two died due to sepsis after catheter-related blood stream infection (nonresponder group), one due to sepsis with urinary tract infection (responder group), three developed pneumonia and died (two in responder group and one in nonresponder group), three due to uremia because of refusal of dialysis (nonresponder), and one had sudden death (responder). The relative risk of death among the nonresponder group was 5.6 (95% CI: 1.96–15.9, p = 0.001).
The overall patient survival was 64.9 months (95% CI: 55.4–74.5). The patient survival in responder group was 75.7 months (95% CI: 67.9–83.4) and was 31.7 months (95% CI: 10.2–53.2) in nonresponders. The survival among the responders was significantly better in the responder group compared to nonresponders by logrank test (p = 0.001). On Kaplan-Meir survival analysis, the patient survival at six months was 88.1% among the responders compared to 85.6% among the nonresponders. No patient died after 12 months, and the patient survival remained same as 88.1% in responder group and 34.2% in nonresponder till the end of follow up (p < 0.001) [Figure 2].
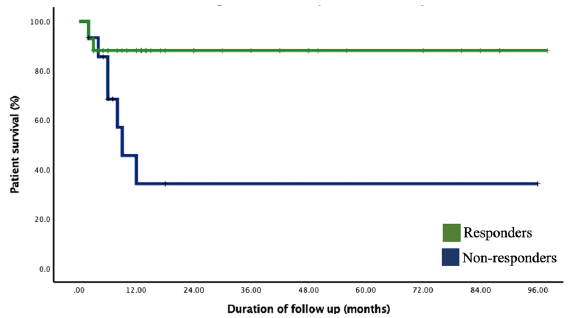
- Kaplan-Meir survival analysis showing the estimated patient survival among the responder versus nonresponder groups.
Patient survival between anti-CFH positive and negative patients
Six patients (18.2%) died in the anti-CFH antibody positive group and six (25%) in the seronegative group (p = 0.53). On the Kaplan-Meir survival analysis [Figure 3], the mean estimated patient survival in the anti-CFH positive and negative groups were similar with 79.1 months (95% CI: 65.4–92.7) in anti-CFH positive and 68.4 months (95% CI: 49.6–87.2) in anti-CFH negative patients (p = 0.45). On the Kaplan Meir survival analysis [Figure 3], during follow-up, the patient survival between anti-CFH positive and negative group at six months was 87.7% versus 87.5%; at 12 months it was 79.6% versus 70.1%, and that remained similar till the end of follow-up. The patient survival compared among the two groups by logrank test was similar (p = 0.43).
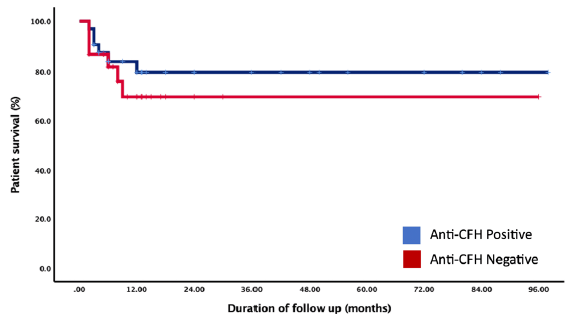
- Kaplan-Meir survival analysis showing the estimated patient survival difference between anti-CFH positive and negative groups of patients. CFH: Complement Factor-H.
Renal survival
Out of the total cohort, 42 individuals (73.6%) achieved initial remission; of them 29 (50.9%) had CR and 13 (22.8%) had PR. On follow-up, a total of 16 (28%) patients developed end-stage renal disease (ESRD) requiring renal replacement therapy, six (18.2%) in anti-CFH positive group, and ten (41.7%) in anti-CFH negative group. The overall mean dialysis-free renal survival was 71.6 months (95% CI: 60.2–83.1). The mean dialysis-free renal survival was 77.2% at six months and remained 71.9% till end of follow-up. The mean dialysis-free renal survival among the anti-CFH positive group was 80 months (95% CI: 67–93), which was significantly better than compared to 48 months (95% CI: 31–65; p = 0.03) seronegative patients. On comparing the anti-CFH positive and negative groups by logrank test, the renal survival was significantly better among anti-CFH seropositive compared to the negative counterparts (p = 0.03). As shown in Figure 4, the dialysis-free renal survival in anti-CFH positive patients versus anti-CFH negative patient was 84.8% versus 79.2%; 81.2% versus 61.5%; and 81.2% versus 55.9% at 6, 12, and 24 months, respectively.
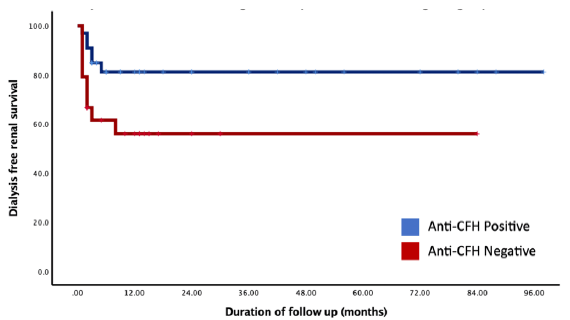
- Kaplan-Meir survival analysis showing the estimated dialysis-free renal survival among patients testing positive for anti-CFH antibodies versus those with anti-CFH negative group. CFH: Complement Factor-H.
Relapse and rehospitalization on follow-up
Out of 55 surviving patients from the initial hospitalization, 21 (38.1%) needed readmission later on. Reasons included relapse in nine (16.4%), hypertensive emergencies in five (9%), serious infections requiring hospitalization in six (10.9%), and one case of acute pancreatitis. Among the 42 initial responders, there were nine relapses. Four of these patients had CR and five had PR during the initial hospital stay. Four relapses had no identifiable trigger, while infections were the cause in three cases. Two relapses were due to noncompliance with medication. Treatment involved optimizing immunosuppression, managing infections, and in six cases, plasmapheresis. Eight out of nine relapses achieved remission again after treatment (four PR from the index PR group, and four CR from the index CR group).
Regarding the six patients with serious infections, two had central line-associated bloodstream infections (CRBSIs), three had pneumonia (including one with COVID-19), and one had a urinary tract infection, all leading to fatalities. Details of mortality and readmissions following the initial hospitalizations are presented in Table 5.
| Variable | n (%) |
|---|---|
| Mortality | 12 (21%) |
| Sepsis | 6 |
| Inadequate dialysis | 3 |
| Intracranial bleed | 2 |
| Sudden cardiac death | 1 |
| Rehospitalizations | 21 (28%) |
| Relapse | 9 (16.4%) |
| No precipitating event | 4 |
| Infectious trigger | 3 |
| Drug default | 2 |
| Serious infections requiring hospitalization | 6 (10.5%) |
| CRBSI | 2 |
| LRTI | 3 |
| UTI | 1 |
| Other indications | |
| Accelerated hypertension | 5 |
| Acute pancreatitis | 1 |
| Anti-hypertensive requirement among the responders, Median (IQR) | 2 (1–4) |
CRBSI: Catheter related blood stream infection, LRTI: Lower respiratory tract infection, UTI: Urinary tract infection, IQR: Interquartile range.
Discussion
In this single center study on aHUS, a rare but a severe disorder of TMA, we observed that the most common cause of aHUS was anti-CFH antibody-associated aHUS followed by complement three mediated dysregulation. The mean age was 16 years and there was a male preponderance (63%), similar to earlier studies.2–4 The outcomes of anti-CFH antibody-associated aHUS was better than those of anti-CFH negative patients. aHUS may be triggered by various factors, including viral or bacterial infections, pregnancy, surgery, and injuries,11,12 as we have also observed in our study. We also observed prodromal symptoms in majority of patients with fever, indicating some form of infection in 42% and diarrheal symptoms in 15.7%. Infections are one of the most important triggering factors for the onset of disease, which was also observed in other studies. In our cohort, one patient had probable association with relapse of the disease, post SARS-CoV-2 infection.19 Diarrheal illnesses in aHUS in our cohort is less than those of reported in the literature in approximately one-third of the patients.20–22 Other clinical manifestations like hypertension, oliguria, and neurological involvement with seizure were similar to the other study.23
One of the most important observations of our study was the association of aHUS with anti-CFH antibodies in the majority, 60% of cases, consistent with findings in other Indian studies.24,25 It was much higher than that reported in Western literature, where seropositivity was noted in only 13%–25%.1,26 The incidence of genetic mutations of complement-regulating genes in the French National Registry was 60%.3 Genetic mutations may be underreported in our study, as all patients have not undergone exome sequencing due to logistic and financial constraints. We have observed that in the scenario of the nonavailability of Eculizumab, the plasmapheresis and immunosuppression strategies provided a fairly good outcome. The overall response rate with our protocol was 72% as compared to 80% with that of Eculizumab-based therapy.27 In spite of guidelines advocating for higher number of plasmapheresis sessions12 in the management of aHUS, our cohort, which underwent a limited number of plasmapheresis sessions, demonstrated a comparable response rate. This suggests that a more restrained approach to immunosuppression, characterized by fewer plasmapheresis sessions, may yield similar response rates while reducing the risk of infection. We observed that female gender, age ≥16 years, presence of seizures, and a baseline anti-CFH ≥4000 were predictors of nonresponsiveness in our studies. The presence of seizures could indicate very severe disease and may be consequential to uncontrolled hypertension and hence could portend poor prognosis.28 Although the presence of anti-CFH antibody seropositive status conferred a good prognosis in terms of response to immunosuppression, a high titre of ≥4000 showed a poor outcome. It is possible that this subgroup with very high titre may have had a higher requirement of immunosuppression. Two patients in our cohort who did not respond and relapse with continued on MMF responded to rituximab therapy. This may call for combined plasma exchange and up-front rituximab in centers that do not have access to Eculizumab. We have not observed any association of treatment response with histological findings, except that the IFTA was significantly high in the group of nonresponder patients, which was the expected finding. The renal outcomes in the anti-CFH antibody-positive group were better than those in negative patients. It is possible that autoantibodies responded better to the removal of plasma after plasmapheresis, and immunosuppression checked the subsequent formation of antibodies, while complement dysregulation could not be checked effectively with immunosuppression. Plasmapheresis may have functioned in such patients by removing some complement and dysregulated protein products from plasma, helping in some response in these groups of patients as well.
In terms of patient survival, the study revealed comparable rates at 6 and 12 months between two groups with and without anti-CFH antibodies.4 This indicates that factors beyond complement dysregulation could be influencing patient survival. The common causes of mortality were sepsis, uncontrolled hypertension, and seizures. Effective control of hypertension and appropriate management of opportunistic infections and seizures may improve patient outcomes. The immunosuppression and plasmapheresis predispose them to the risk of infection. These patients require close follow-up. All recorded deaths, whether among responders or nonresponders, happened within the initial 12 months of disease onset. This pattern strongly implies that heightened disease activity or infections following immunosuppression, aimed at managing disease activity, likely played a significant role in mortality. About 40% of the patients in our study needed readmission and 11% had infections that required hospitalization. Effective control of BP and prompt treatment of infections with appropriate antibiotics is key to successful patient outcomes. Relapses are not uncommon and nearly one in five patients had a relapse in this study. Patients who had relapse had dismal prognosis on account of severity of disease as well as higher rate of infections and sepsis due to higher cumulative need of immunosuppression.
Large, single-center study with availability of long-term outcome data of a uniform management protocol in patients with aHUS: Eculizumab, the standard of care, was not given to any patient. The study supports the use of a reasonably economical regimen, involving plasma exchange and immunosuppression with favorable short- and long-term outcomes. This proves to be a viable alternative in resource-limited settings where Eculizumab is unavailable. The retrospective design and single-center study remain major limitations of the study. The mutational analysis could not be performed in all patients.
Anti-CFH antibodies remain a major cause of aHUS in our cohort. Anti-CFH-associated aHUS has better renal outcomes than negative patients. The patient survival remains similar between the two groups. Plasma exchange with immunosuppression may be used as a viable alternative regimen to treat the patients if Eculizumab is not available.
Data availability
The data is available with first and corresponding authors. It can be made available with a reasonable request. However, it may not be made public because of ethical issues.
Conflicts of interest
There are no conflicts of interest.
References
- Incidence and cost of haemolytic uraemic syndrome in urban China: A national population-based analysis. BMC Nephrol. 2022;23:122.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94:408-18.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics and outcome of atypical hemolytic uremic syndrome: A nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics and outcome of Canadian patients diagnosed with atypical hemolytic uremic syndrome. Can J Kidney Health Dis. 2020;7:2054358119897229.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15-39.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312-22.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic criteria for atypical hemolytic uremic syndrome proposed by the joint committee of the Japanese society of nephrology and the Japan pediatric society. Clin Exp Nephrol. 2014;18:4-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20:536-43.
- [CrossRef] [PubMed] [Google Scholar]
- Working party from the renal association, the British committee for standards in haematology and the British transplantation society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol. 2010;148:37-47.
- [CrossRef] [PubMed] [Google Scholar]
- An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2015;35:421-47.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48:624-36.
- [CrossRef] [PubMed] [Google Scholar]
- Hemolytic uremic syndrome in a developing country: Consensus guidelines. Pediatr Nephrol. 2019;34:1465-82.
- [CrossRef] [PubMed] [Google Scholar]
- Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 2014;85:1151-60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and immunological profile of anti-factor h antibody associated atypical hemolytic uremic syndrome: A nationwide database. Front Immunol. 2019;10:1282.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312-22.
- [CrossRef] [PubMed] [Google Scholar]
- KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-84.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertension in children and adolescents: Epidemiology and natural history. Pediatr Nephrol. 2010;25:1219-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560-72.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 vaccination and atypical hemolytic uremic syndrome. Front Immunol. 2022;13:1056153.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Atypical hemolytic uremic syndrome triggered by varicella infection. IDCases. 2017;9:89-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Aortic valve replacement as a trigger of atypical hemolytic uremic syndrome. Ann Thorac Surg. 2017;104:e255-6.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical hemolytic uremic syndrome and nephrotic syndrome associated with cytomegalovirus infection. Nephron. 2021;145:188-91.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of atypical hemolytic uremic syndrome in children: Single centre experience. Indian J Hematol Blood Transfus. 2014;30:342-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Characterization of genetic predisposition and autoantibody profile in atypical haemolytic-uraemic syndrome. Immunology. 2018;154:663-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-factor H antibody and its role in atypical hemolytic uremic syndrome. Front Immunol. 2022;13:931210.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555-63.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS) BMJ Open. 2013;3:e003573.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Extra-renal manifestations of atypical hemolytic uremic syndrome. Pediatr Nephrol. 2019;34:1337-48.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








