Translate this page into:
Membranous Nephropathy with Rapid Progression
Address for correspondence: Dr. K. Thirumal Valavan, Department of Nephrology, Government Stanley Medical College and Hospital, Old Jail Road, Chennai - 600 001, Tamil Nadu, India. E-mail: thirumalvalavan97@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We report a 49-year-old man with microscopic hematuria, subnephrotic proteinuria, and rapidly progressive renal failure. His biopsy had features of PhosphoLipase A2 Receptor (PLA2R) positive membranous nephropathy with circumferential cellular crescents. Further work-up revealed IgG antiGlomerular Basement Membrane (anti-GBM) antibody titer of 188 U/mL (normal <7 U/mL). A final diagnosis of membranous nephropathy with anti-GBM disease was made. These two distinct pathological entities can occur together resulting in significant morbidity and mortality unless diagnosed early and treatment initiated promptly. Outcomes have been poor, given the nonspecific presentation and delay in diagnosis.
Keywords
Anti-GBM
crescentic transformation
membranous nephropathy
Introduction
The clinical course of membranous nephropathy, though characterized by gradual progression, can have rapid deterioration of renal function.[12] Acute interstitial nephritis, malignant hypertension, renal vein thrombosis, and crescentic transformation being the causes for such rapid decline.[12] Antiglomerular basement membrane (anti-GBM) disease can co-occur, precede, or succeed membranous nephropathy onset in a given patient.[3] The prognosis of the dual glomerulopathy has been dismal despite therapy, unless the crescentic process is identified early.
Case Report
A 49-year-old man, carpenter by occupation, without any comorbidities presented with nausea and anorexia of 1 month duration in July 2018. A basic investigation showed a serum creatinine of 2.4 mg/dL and was advised a nephrology consult. However, he consulted only after a period of 17 days, since he did not have severe symptoms. He had no history of rash, hematuria, oliguria, cough, hemoptysis, or fever. He denied native medicine or over the counter medicine intake. Examination revealed mild pedal edema and blood pressure of 150/80 mm Hg. Investigations revealed urine albumin 3+ and 20–25 RBCs/HPF, 24 hour urine protein of 2.6 g, hemoglobin 9.2 g/dL, platelet count 1.8 lakhs, serum creatinine 11.3 mg/dL, albumin 3.1 g/dL, and echogenic normal-sized kidneys on ultrasonography. His complement levels were normal and antinuclear antibody was negative. In view of rapidly progressive glomerulonephritis, he was initiated on hemodialysis and administered pulse methyl prednisolone (three doses of 15 mg/kg) followed by renal biopsy. Of the 14 glomeruli, four were sclerosed and five glomeruli had circumferential cellular crescents compressing the glomerular tuft [Figure 1a]. The normal glomeruli showed uniform capillary wall thickening with spike and pinhole lesions on Periodic Acid Schiff (PAS)-silver stain, without endocapillary or mesangial proliferation [Figure 1b]. Interstitial edema with scattered lymphoplasmacytic infiltrate, tubular epithelial injury, and mild arterial medial hyperplasia were present. Interstitial fibrosis with tubular atrophy was about 10%–15%. Immunofluorescence showed capillary loop granular positivity for IgG 3+ and C3 3+ [Figure 2]. There was no light chain restriction. Immunostaining for IgA, IgM, and C1q were negative. Tissue staining for PLA2R was intensely positive [Figure 3]. Further investigations showed anti-GBM titer of 188 U/mL and cytoplasmic-Anti-Neutrophil Cytoplasmic Antibodies (ANCA) and perinuclear-ANCA titers were negative. Serology for HIV, Hepatitis B, and Hepatitis C were negative. An age appropriate malignancy screening was also done. Screening CT of chest and abdomen were normal. Esophagoduodenoscopy showed laxity of esophagogastric junction with gastric antral erosions while colonoscopy was normal. His prostate specific antigen levels were within normal limits. A final diagnosis of primary membranous nephropathy with anti-GBM disease was made. All crescents being cellular without significant chronicity, he was administered intravenous cyclophosphamide and six sessions of therapeutic plasma exchange. During his period of treatment, he developed significant pedal edema and became anuric. Since he continued to be dialysis dependent even at the end of three months, it was decided to discontinue immunosuppressive therapy.
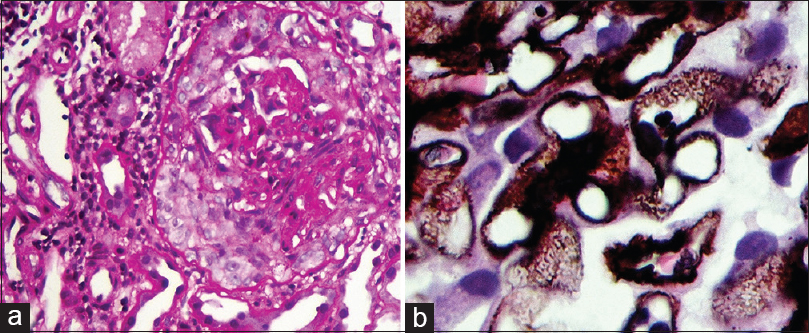
- (a) Cellular crescent encircles and compresses the capillary tuft (Periodic Acid Schiff × 400 magnification). (b) PAS-silver stain showing spike lesions in the GBM
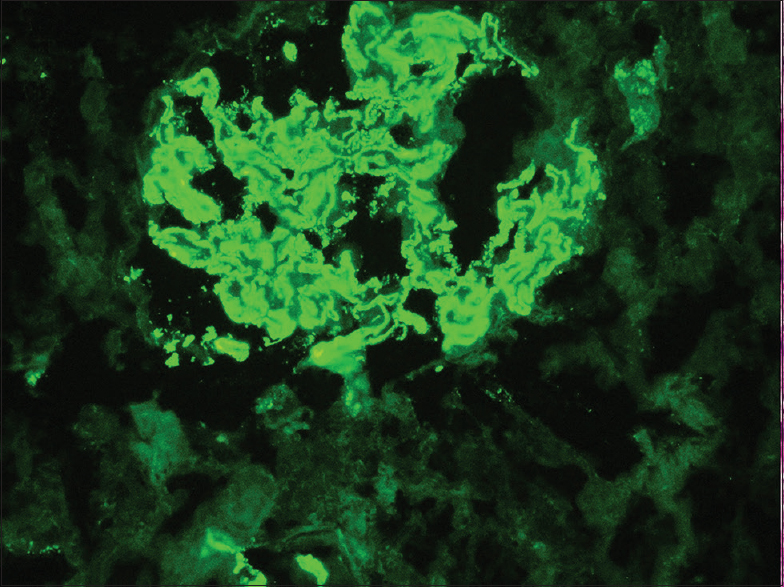
- Immunofluorescence showing intense granular staining for IgG antisera along the capillary loops
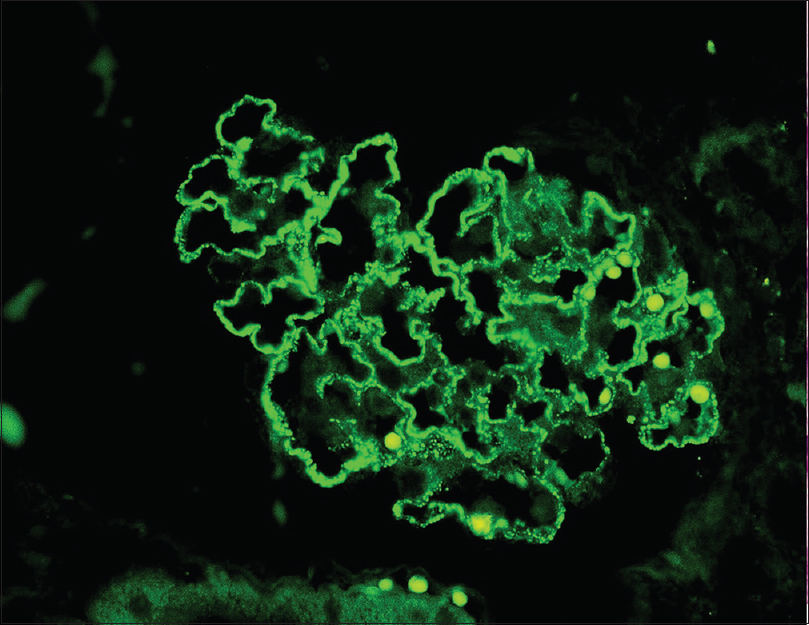
- Immunofluorescence showing intense staining for PLA2R
Discussion
Membranous nephropathy can be either primary or secondary to a variety of conditions including autoimmune diseases, drugs, chronic infections, or malignancies.[123] When crescents are observed in membranous nephropathy, the diagnosis is usually a class III/IV with class V membranous lupus nephritis.[23] Crescentic transformation could also be due to anti-GBM disease, ANCA-associated glomerulonephritis, or very rarely idiopathic.[345] In our patient, the occurrence of crescents with elevated IgG anti-GBM antibody titer in a picture, otherwise consistent with membranous nephropathy, led us to suspect superimposed anti-GBM disease. Prior case reports have demonstrated liner IgG staining in such cases to suggest anti-GBM disease, which was not present in our patient. However, the coarse granular staining seen in membranous lesions may mask the linear IgG pattern of anti-GBM disease.[256]
Since the first case report of anti-GBM disease was diagnosed post mortem in a patient with membranous nephropathy by Klassen et al., cases of the two diseases occurring together have been reported.[37] Either of the two can precede the other, or both can occur simultaneously. To the best of our knowledge, only 34 cases of this dual glomerulopathy have been reported in literature till 2014.[28] When anti-GBM disease preceded, the patients were usually younger and had features of hematuria, oliguria, or hemoptysis and while membranous nephropathy preceded, the patients tend to be elderly adults and presented with features of edema.[29] Though our patient presented with nausea and loss of appetite without edema, biopsy features suggestive of class II-III membranous nephropathy suggest membranous nephropathy to have preceded anti-GBM disease, although it is not conclusive. Does the co-occurrence of the two disease entities a co-incidence or represent a pathological association? Few animal models have come in support of their association. In the Brown–Norway rat mercuric model described by Druet et al., granular capillary wall IgG deposition was preceded by linear IgG pattern, whereas in the membranous nephropathy model of DBA/1 mice induced by immunization with α3 (IV) noncollagenous 1 domain, it was observed that the immune deposits tend to be subepithelial before incorporating into the GBM.[10] Hypotheses to explain the pathogenesis of the association have not been conclusively proven. Anti-GBM disease is a “conformopathy” arising due to conformational change in the α3, α4, α5 quaternary structure of the GBM exposing cryptic epitopes and it has been speculated that the immune-mediated damage to the GBM in membranous nephropathy might expose these “hidden” epitopes resulting in anti-GBM disease.[259] Conversely, anti-GBM antibodies might cause podocyte injury releasing antigens, forming immune complexes and culminating in membranous nephropathy.[29] Regarding the prognosis, literature review shows that the dual glomerulopathy, despite treatment, usually portends a poor prognosis with most patients either remain dialysis dependent or die, with some authors claiming anti-GBM preceding membranous nephropathy to have a good renal prognosis, compared to poorer outcome if the order of occurrence was reversed.[87] Jia et al. showed that dual glomerulopathy patients fared better in terms of renal prognosis when compared to anti-GBM disease alone and that antibodies in patients with isolated anti-GBM disease had a broader reactivity to all collagen α chain protomers, whereas antibodies from patients with dual disease had a narrower spectrum of reactivity, mostly to α3 chain.[8] He claimed the difference in prognosis was related to the antigenic reactivity spectrum. The bottom line of all these case reports is that those patients who fared better had a lesser degree of creatinine rise and it is a well-known fact that glomerular filtration rate at diagnosis is a predictor of outcome in crescentic glomerulonephritis.[9] So, as crescentic transformation can wreak havoc in an innocuous membranous nephropathy course, at least in established membranous nephropathy patients, regular surveillance and patient education can help in identifying this rare catastrophe early and may help in improving the disease outcome especially in terms of dialysis independency.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge Dr. Anila Abraham, consultant pathologist, Renopath, Center for Renal and Urological Pathology Pvt., Ltd., for helping us with biopsy interpretation.
References
- Membranous nephropathy with crescents in a patient with Hashimoto's thyroiditis: A case report. Medicine. 2014;93:e63.
- [Google Scholar]
- Membranous nephropathy with crescents: A series of 19 cases. Am J Kidney Dis. 2014;64:66-73.
- [Google Scholar]
- Crescentic glomerulonephritis and membranous nephropathy: A rare coexistence. Int Urol Nephrol. 2015;47:1373-7.
- [Google Scholar]
- Transformation of membranous into anti-GBM nephritis. Indian J Nephrol. 2012;22:370-3.
- [Google Scholar]
- Membranous glomerulopathy with superimposed pauci-immune necrotizing crescentic glomerulonephritis. Clin Kidney J. 2012;5:587-90.
- [Google Scholar]
- Coexistence of anti-glomerular basement membrane glomerulonephritis and membranous nephropathy in a female patient with preserved renal function. Tohoku J Exp Med. 2017;243:335-41.
- [Google Scholar]
- The clinical andimmunological features of patients with combined anti-glomerular basement membrane disease and membranous nephropathy. Kidney Int. 2014;85:945-52.
- [Google Scholar]
- Anti-glomerular basement membrane disease superimposed on membranous nephropathy: A case report and review of the literature. J Med Case Rep. 2010;4:237.
- [Google Scholar]
- Conversion of goodpasture's syndrome into membranous glomerulonephritis. Nephrol Dial Transplant. 2001;16:2082-5.
- [Google Scholar]







