Translate this page into:
Mucormycosis of the Thyroid Gland: A Cataclysmic Event in Renal Allograft Recipient
Address for correspondence: Dr. N. Prasad, Department of Nephrology, SGPGIMS, Lucknow, Uttar Pradesh, India. E-mail: narayan.nephro@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Invasive fungal infection is a complication seen in immunocompromised patients. A disseminated fungal infection has a high rate of mortality. Although disseminated infection is known to be seen in most organs, thyroid involvement is rarely reported. Hence, we report a fatal case of thyroid mucormycosis which resulted into laryngeal nerve paralysis and death of a renal allograft recipient.
Keywords
Airway
mucormycosis
renal allograft
thyroid
Introduction
Immunocompromised patients are prone to various types of infections. This group of patients is especially prone for atypical manifestations of common infections, opportunistic infections, atypical sites of infections, and occult infections, all leading to a missed or a delayed diagnosis. Invasive fungal infection (IFI) has been increasing in incidence in this group of patients as more intensive chemotherapy/immunosuppressive therapy is used in expectation for a cure or finer control of the disease.[1] We report a renal allograft recipient who had recurrence of focal segmental glomerulosclerosis (FSGS) and developed acute fungal thyroiditis caused by mucormycosis. He subsequently died because of bilateral vocal cord palsy due to bilateral laryngeal nerve involvement.
Case Report
A 52-year-old male, renal allograft recipient, presented to the transplant clinic with complaints of fever, nonproductive cough, and throat pain for 2 days. His brief pretransplant course suggested native kidney disease as chronic glomerulonephritis as he had a history of significant proteinuria and hypertension in the past. He underwent living donor renal transplant with his haploidentical brother as donor with basiliximab induction and triple immunosuppressant (prednisolone, tacrolimus, and mycophenolate mofetil) in February 2013. On the postoperative day 8, he had an increase in serum creatinine from 1.2 to 1.8 mg/dl and graft biopsy revealed borderline rejection. He was treated with intravenous methylprednisolone. He was discharged on maintenance immunosuppression (prednisolone, tacrolimus, and mycophenolate mofetil) with a baseline serum creatinine of 1.2 mg/dl. On the 62nd day, he presented to the transplant clinic with new onset edema. Evaluation revealed graft dysfunction (serum creatinine: 2.1 mg/dl) and a proteinuria of 26 g/day/1.73 m2. His second allograft biopsy revealed FSGS [Figure 1] without any deposit of immunoglobulin and complement on immunofluorescence, and electron microscopy showed diffuse foot process effacement. With FSGS presumed to be the native kidney disease, he was considered to have recurrence of FSGS. He was started on 3 times per week plasmapheresis. His proteinuria decreased to 14 g/day and serum creatinine remained stable at 1.5–1.8 mg/dl. He was given 4 doses of rituximab at 500 mg per week and switched over to once a week plasmapheresis. His serum creatinine remained stable (1.45–1.7 mg/dl) and proteinuria decreased (3.9–4.1 g/day). Maintenance immunosuppression was changed from tacrolimus to cyclosporine, along with mycophenolate and prednisolone. The reason of changing from tacrolimus to cyclosporine was few literature evidence of better response of recurrence of FSGS with cyclosporine.[2] Every time, his serum creatinine increased to 1.68–1.9 mg/dl preplasmapheresis, only to decrease after plasmapheresis to 1.19–1.32 mg/dl. He remained plasmapheresis dependent. The patient was noncompliant and was on 1 plasmapheresis in 2 weeks. In February 2014, he had a creeping increase in serum creatinine (peak value: 3.2 mg/dl) and proteinuria increased from baseline of 4.1 to 7.5 g. He underwent third allograft biopsy and was opined as FSGS. As there was only mild interstitial fibrosis and tubular atrophy in the present biopsy, his plasmapheresis dose was escalated to 3 per week. However, his graft dysfunction persisted. With the 3rd graft biopsy being CD80 positive, he received 2 doses of abatacept 500 mg each at 3-week interval. His right radiocephalic arteriovenous fistula (AVF) had thrombosis and high radial AVF was made, with interim plasmapheresis done through femoral access, to resume later from the right high radial AVF. He was on maintenance plasmapheresis twice a month, until this admission in July 2014 (with serum creatinine at 1.9–2.2 mg/dl with 3.6–4.0 g/day proteinuria).

- Light microscopy of renal allograft showing synechiae formation (thin arrow) and segmental sclerosis (thick arrow) (Periodic acid–Schiff, ×200)
At the office visit for his fever and cough, he was given symptomatic medications along with amoxicillin and clavulanate potassium. He reported back next day for indoor admission in view of no improvement. His clinical examination was unremarkable except for temperature of 101.8°F. Oropharyngeal examination did not show any membrane or thrush. No lymph node enlargement was noted. His urine and blood cultures were sterile. Computed tomography (CT) of the chest and paranasal sinuses was normal. He was started on intravenous amoxicillin and clavulanate potassium. On day 3, the patient complained of swelling in the neck. Examination showed swelling which moved with deglutition. Ultrasound showed heterogeneous echotexture of bilateral lower part of thyroid and isthmus and multiple hypoechoic lesion [Figure 2a and b]. Ultrasound guided fine-needle aspiration was done and cytology revealed thyroid tissue with lymphocytic infiltration. On day 4, he developed hoarseness of voice. Immediate repeat ultrasound did not show any increase in the swelling size or hematoma. Laryngoscopy showed right vocal cord palsy [Figure 2c]. The patient had no complaints of dyspnea or any choking sensation. After 6 h, the patient had a sudden onset stridor and dyspnea. Examination showed bilateral vocal cord palsy and mild laryngeal edema. An urgent tracheostomy and ventilation was planned. He developed cardiac arrest during the same and could not be revived from it. The family consented for a postmortem biopsy of the thyroid gland. Biopsy showed mucormycosis of thyroid gland with severe angioinvasion [Figure 3].

- (a and b) Ultrasonogram of thyroid with hypoechoic lesions and (c) laryngoscopy picture showing paramedian-fixed right vocal cord indicating vocal cord palsy
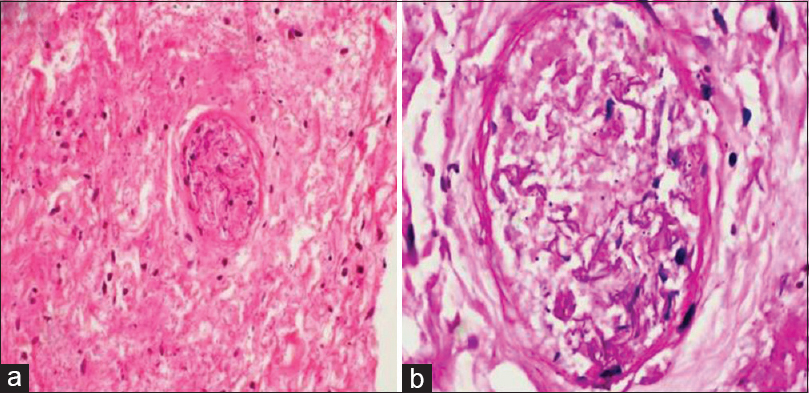
- Section from postmortem thyroid biopsy shows coagulative necrosis of thyroid parenchyma with presence of angioinvasive mucormycosis showing nonseptate hyaline fungal hyphae occluding the blood vessel (a: H and E, ×200; b: Periodic acid Schiff, ×400)
Discussion
In this case report, we observed that a renal transplant recipient had recurrence of FSGS who responded partially with plasmapheresis and multiple immunosuppressants, rituximab, intravenous immunoglobulin, and abatacept; however, the patient died because of mucormycosis of thyroid gland. This emphasizes that there should be critical balance between immunosuppression and infection for success of transplantation.
IFI is known to complicate the course of many immunocompromised patients, like patients with uncontrolled diabetics, with leukemia, lymphoma, AIDS, and use of immunosuppressive therapy. IFIs after renal transplantation are uncommon, incidence ranging from 1% to 14% in renal transplant recipients, with mucormycosis representing only in 0.2%–1.2% of cases.[1] IFI is reported to involve most of the organs; however, fungal invasion of thyroid is very rare. The possible explanation of less thyroid involvement as compared to that of other organs could be its rich blood supply and lymphatic drainage. It is also separated from other structures by its fibrous capsule and fascias of the neck. Iodine in the thyroid gland could also act as an antimicrobial agent; however, it is less likely to be a factor as the concentration of 3 μg/g of thyroid is not proven to have antimicrobial activity.[34]
Moreover, the thyroid involvement could be because of hematogenous or lymphatic spread. Usually, the thyroid involvement is a part of disseminated involvement. However, rarely isolated thyroid involvement is reported with histoplasmosis, Aspergillus, coccidioidomycosis, and pneumocystis infection.[4] Infection without systemic involvement could be attributed to seeding during transient periods of fungemia. In our case, the focus of the dissemination remains obscure. Clinical examination and the CT scan of the chest and sinuses were normal. We presume that thyroid was seeded during fungemia, akin to the description in patients with isolated allograft mucormycosis.[5] However, his blood culture was sterile. The other possible route could be direct invasion through skin injury in thyroid region during shaving, a very remote possibility; however, the patient had denied any such history of injury.
Rarely, candida, cryptococcal, coccidioidal, and histoplasma thyroiditis is reported as a part of disseminated disease. A recent review by Goldani et al.[6] revealed Aspergillus as one of the most common fungal infections of the thyroid. Cases of aspergillus thyroiditis after dissemination,[7] fatal aspergillus thyroiditis with tracheal involvement,[8] and esophageal involvement with stenosis and obstruction[9] have been reported in nontransplant recipients.[789] A rapid onset of hoarseness, compressive symptoms, and dyspnea at rest due to aspergillus thyroiditis has also been reported in renal transplant recipients who required urgent tracheostomy.[10]
Thyroid involvement by mucormycosis is rarely reported particularly in renal transplant recipients. Recent review reports only 10 cases of thyroid involvement and only 1 among them was a renal allograft recipient.[11] He had a disseminated mucormycosis and died due to sepsis.[211] The rhinocerebral, pulmonary, and gastrointestinal forms are the common forms of mucormycosis. It can also involve the liver, genitourinary system, and the skin. Clinically, local signs and symptoms of fungal thyroiditis are indistinguishable from other infectious thyroiditis. Disseminated infection is defined as infection at two or more noncontiguous sites. In the patients with solid organ transplant, disseminated disease occurs in 9%–26% of cases, with the highest incidence among liver transplant recipients (26%–55%) and lowest in kidney transplant recipients (9%–13%).[1213] Reason for this disparity among different organ transplant groups is not known. In mucormycosis, about 50% of patients with pulmonary infection, 38% with gastrointestinal infection, and 20% with cutaneous infections suffered from dissemination. Disseminated infection in immunocompromised patients can be asymptomatic in the initial period. Nearly half of the patients with thyroid involvement remained asymptomatic even when autopsy reveals significant areas of fungal infiltration and necrosis.[12] In addition to sparse case reports, thyroid involvement by fungal infection is reported in many autopsy series of immunocompromised patients. In an autopsy series of 21 patients with disseminated Cunninghamella bertholletiae infection, thyroid gland involvement was seen in 19%. Among 9 cases of disseminated Rhizomucor pusillus infection, thyroid involvement was seen in 22%. Hence, this may be indicative of a missed antemortem diagnosis as many of them may have remained asymptomatic.[1415] Our patient was unique in the sense that he did not reveal any evidences of mucor infections at common site such as rhinocerebral and pulmonary involvement. He had initially unilateral vocal cord paralysis and fine-needle aspiration cytology of the thyroid did not reveal any microorganisms but lymphocytic thyroiditis.
Our patient was highly immunosuppressed with plasmapheresis, rituximab, and abatacept in the past to treat recurrence of FSGS, only with partial benefit. Abatacept was recently reported to be beneficial in recurrence of FSGS in CD80-positive patients.[16] These immunosuppression might have predisposed him to this infection. However, the cause of death in our case remains obscure. When the patient was tried for endotracheal intubation, he was noted to have bilateral vocal cord palsy and laryngeal edema, so he underwent urgent tracheostomy. Mucormycosis is an angioinvasive fungus. The biopsy [Figure 1] shows the fungal ball in the blood vessel. The patient had unilateral vocal cord palsy, and within 6 h, he had bilateral palsy. Rapid blood vessel invasion could have led to bilateral recurrent laryngeal nerve involvement with vocal cord palsy and death.
Conclusion
Mucor infection to thyroid may cause rapid vasoinvasion and laryngeal nerve palsy leading to death of the patient. A simple fine-needle aspiration may miss the diagnosis. A strong suspicion and Trucut biopsy is required to diagnose this unusual site of dissemination.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Two decades of experience in mucormycosis after kidney transplantation. Ann Transplant. 2011;16:44-8.
- [Google Scholar]
- High-dose oral cyclosporin therapy for recurrent focal segmental glomerulosclerosis in children. Am J Kidney Dis. 2004;44:50-6.
- [Google Scholar]
- Determination of selenium and iodine in human thyroids. J Trace Elem Med Biol. 2005;19:23-7.
- [Google Scholar]
- Disseminated zygomycosis presenting as thyroid abscess in a renal allograft recipient. Nephrol Dial Transplant. 2007;22:641-4.
- [Google Scholar]
- Isolated zygomycosis in a bought living unrelated kidney transplant. Transpl Int. 1996;9:600-2.
- [Google Scholar]
- Fatal airway obstruction caused by invasive aspergillosis of the thyroid gland. Leuk Lymphoma. 2002;43:669-71.
- [Google Scholar]
- Purulent thyroiditis - A clinical study of 5 cases. Acta Chir Belg. 2009;109:618-9.
- [Google Scholar]
- Aspergillus fumigatus abscesses of the thyroid with obstruction of the esophagus. Thyroid. 2004;14:786-8.
- [Google Scholar]
- A case of disseminated aspergillosis with thyroid involvement. Eur J Clin Microbiol Infect Dis. 2001;20:358-9.
- [Google Scholar]
- Hyperthyroidism secondary to disseminated mucormycosis in a child with acute lymphoblastic leukemia: Case report and a review of published reports. Mycopathologia. 2013;175:123-7.
- [Google Scholar]
- Aspergillus thyroiditis: A review of the literature to highlight clinical challenges. Eur J Clin Microbiol Infect Dis. 2012;31:3259-64.
- [Google Scholar]
- Mucormycosis in a renal transplant recipient: Case report and comprehensive review of literature. Int J Nephrol 2014. 2014;950643
- [Google Scholar]
- Postmortem analysis of invasive aspergillosis in a tertiary care hospital. Eur J Clin Microbiol Infect Dis. 1997;16:1-6.
- [Google Scholar]
- Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. 2011;24:411-45.
- [Google Scholar]
- Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416-23.
- [Google Scholar]







