Translate this page into:
Mycophenolate versus Cyclophosphamide for Lupus Nephritis
Address for correspondence: Dr. M. Sahay, 6-3-852/A, Ameerpet, Hyderabad - 500 016, Telangana, India. E-mail: drmanishasahay@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Systemic lupus erythematosus is common in our country, and renal involvement is an important cause of chronic kidney disease. This study was aimed at comparing the three regimens, i.e., cyclophosphamide-based regimes (low dose and high dose) and mycophenolate mofetil (MMF)-based regime and determining if cyclophosphamide (CPM)-based regime can be an effective, safe, and cheap alternative to MMF-based regime in a resource-limited setting. Out of 144 patients, females constituted 89%. Nephrotic nephritic presentation was the most common. Rapidly progressive renal failure was seen in in 42 (29.1%) patients. Class IV was the most common 66 (45.8%) histological class. Crescentic glomerulonephritis was seen in 18 (12.5%). Overall remission (complete + partial) at 6 months was seen in 71.4% in National Institute of Health regime, 65% in European lupus nephritis trial protocol and 72.9% in MMF regime. End-stage renal disease and switching to other therapies were comparable among the three groups. Although infections were more with CPM, the difference was not statistically significant. CPM-based therapies were associated with a significantly lower cost.
Keywords
Cyclophosphamide
induction
lupus nephritis
mycophenolate
systemic lupus erythematous
treatment
Introduction
Nephritis is the most severe manifestation of lupus.[1] Before the 1950s, there was no treatment for severe lupus nephritis (LN). Between 1950s and 1970s, corticosteroids were used for the treatment of LN. Introduction of the National Institute of Health (NIH) protocol by Austin et al. in the 1980s, with a combination of immunosuppressives such as steroids and cyclophosphamide (CPM), improved the outcome dramatically with 5 years survival increasing from 17% to 80%.[2] Subsequently, intravenous (IV) CPM became the standard of care in induction regimes.[345] However, IV CPM was associated with complications such as bladder toxicity, gonadal problems, and infections. To reduce the toxicity, low-dose IV CPM was introduced by Houssiau et al. (European lupus nephritis trial [ELNT]). ELNT regime was an attenuated regimen of IV CPM (using six biweekly fixed IV doses of 500 mg IV CPM) and it showed equivalent efficacy and less side effects compared with the NIH regimen.[6] The efficacy of ELNT regimen was confirmed in a subsequent 10-year follow-up study. The ELNT regimen reduced the average total induction dose of IV CPM from 8.5 to 3.0 g. At about the same time, mycophenolate mofetil (MMF) was introduced as an alternative agent for induction by Chan et al.[7] Since then many trials have been done comparing the efficacy and side effects of MMF, NIH regime, and ELNT regime.[89] Among efficacy parameters, the endpoints studied were attaining complete remission and partial remission or progression to end-stage kidney disease (ESKD). The adverse events including infections, deaths, gastrointestinal side effects and hematological side effects were also compared. There was a variation in response to the three regimes in different geographical areas. Although the disease is more prevalent in Asian countries, trials are lacking from these countries to provide effective guidelines in these areas. Hence, this study was carried out to compare the efficacy and adverse effect profile of the three protocols in Indian population.
Aim
The present study was done to compare the efficacy and side effects of the three treatment protocols (ELNT, NIH, and MMF) for LN.
Methods
Patients with proven systemic lupus erythematosus (SLE) and LN as per standard guidelines[101112] were biopsied and classified as per International Society of Nephrology Renal Pathology Society classification.[1314] This prospective study was conducted on patients who met the following inclusion criteria - Those with biopsies showing features consistent with LN Class III, IV either isolated or in combination with Class V. Patients with nonproliferative lesions, i.e., Class I, II, and pure V, those with Class VI LN and those not willing to give informed consent were excluded. Patients were randomly allotted to one of the three treatment protocols by simple randomization. The clinical details were noted. The mode of presentation-nephritic or nephrotic syndrome or rapidly progressive renal failure (RPRF) was noted. SLE Disease Activity Index (SLEDAI) score of the patient at presentation was measured.[15] Patients’ obstetric history was recorded. Induction therapy was initiated with the NIH, ELNT, or MMF protocol.[16] ELNT regime consisted of IV CPM using six biweekly fixed IV doses of 500 mg IV CPM while NIH regime consisted of 0.5 g/m2 monthly for 6 months’ therapy. Injection methyl prednisolone was given in a dose of 500 mg/m2 for 3 days. Subsequently, oral steroids were used in a dose of 1 mg/kg. The steroids were tapered to 10 mg by the end of 3 months. This dose was continued. MMF was given in a dose of 1200 mg/m2. Subsequent maintenance therapy for all patients was with azathioprine (2–3 mg/kg body weight) in all the groups. All patients received hydroxychloroquin in a dose of 6 mg/kg/day. Patients were analyzed in terms of their treatment response and side effects.
Treatment response was measured by assessment of proteinuria and serum creatinine (sCr) at regular intervals. Response to therapy in LN was assessed as complete response (CR), partial response (PR), or no response, end-stage renal disease (ESRD), death or switch to other therapies at 6 months.
Outcome measures were defined as per the Kidney Disease: Improving Global Outcomes guidelines.[12]
CR: Return of SCr to the previous baseline, plus a decline in the urinary protein-to-creatinine ratio (uPCR) to <500 mg/g. PR: Stabilization (±25%), or improvement of SCr, but not to normal, plus a >50% decrease in uPCR. If there was nephrotic-range proteinuria (uPCR >3000 mg/g), improvement required a >50% reduction in uPCR, and an uPCR <3000 mg/g. Deterioration was defined as a sustained 25% increase in SCr.
The side effects during treatment were recorded.
The laboratory parameters for these patients were collected. Serological marker of SLE, i.e., antinuclear antibody (ANA) – was done for diagnosis by biochip indirect immunofluorescence using Hep 2 cells of primate liver. Anti-dsDNA and complement were measured to ascertain activity. Anti-dsDNA was done by biochip indirect immunofluorescence using Crithidia luciliae. Complement was measured by nephelometry.
The data were statistically analyzed. Continuous data were presented as mean ± standard deviation. Normally distributed data were compared using ANOVA. Qualitative data were described as frequencies. Proportions were compared using Chi-square or Fischer exact test as applicable. P < 0.05 was considered statistically significant. All calculations were performed using IBM SPSS 21 Chicago IL USA. The study was approved by the Institutional Ethics Committee.
Results
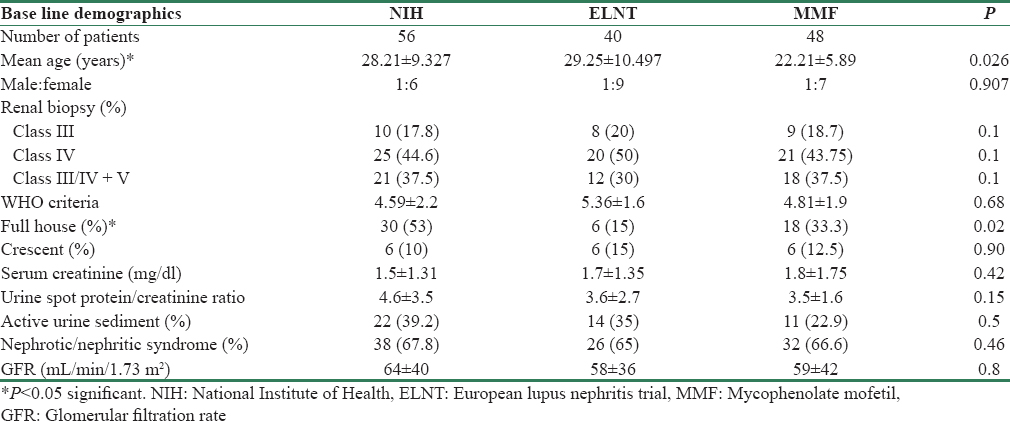
There were 144 patients in our study. Majority belonged to the 21–30 years age group [Figure 1]. There were 128 (89%) females and 16 (11%) males. ANA was positive in 81.8% of the individuals, dsDNA was positive in the 76.4%. The baseline demographics were comparable among the three groups, as shown in Table 1, except for age which was lower in the MMF group.
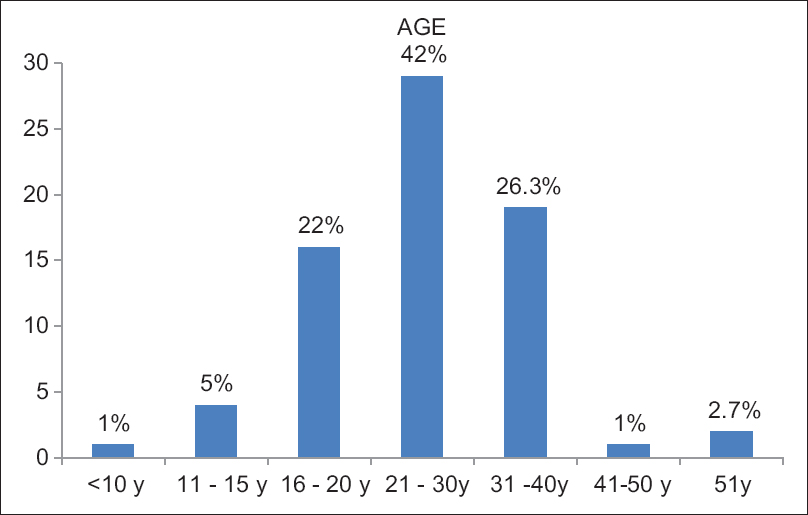
- Age distribution
Among extra-renal manifestations, arthritis was the common manifestation. At presentation 37.5% were hypertensive. Among the various presentations, nephrotic nephritic presentation was the most common. RPRF was seen in 42 (29.1%) patients out of which 21 patients needed dialysis. The mean glomerular filtration rate (GFR) in our study was 61 ± 39 ml/min/1.72 m2. The mean SLEDAI at presentation was 25.3 ± 14.7.
Class IV was the most common 66 (45.8%). Class V with proliferation (III/IV) was seen in 51 (35.4%) patients whereas Class III was seen in 27 (18.75%). Crescentic glomerulonephritis (GN) was seen in 18 (12.5%) patients. Full house immunofluorescence staining on histology was noted in 52 (36.1%). MMF dose varied from 1.5 to 2 g/day. Mean dose of MMF was 1.8 ± 0.1 g/day. Patients came for follow-up once in 2 months. At each visit, urine protein and sCr was done. dsDNA and complement were done at the beginning and at 6 months.
Outcome at the end of 6 months was comparable (P = 0.90) between the three groups, as shown in Table 2. Extrarenal organs involved were skin in 22, nervous system in 14, pancreas (pancreatitis) and liver (autoimmune hepatitis) in 2 patients each.
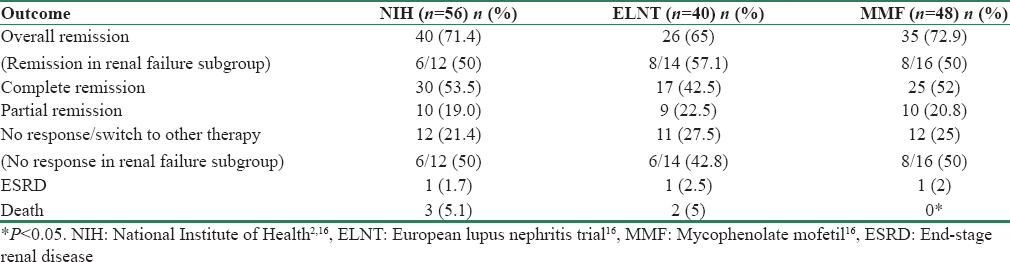
Death and ESRD were comparable among the three groups (P = 0.57). Three deaths were due to extrarenal manifestations of the disease. One patient died because of severe necrotizing pancreatitis, another patient developed autoimmune hepatitis progressing to cirrhosis and expired, and third patient had cardiomyopathy. Two deaths were due to sepsis. Two of the three deaths in NIH group were within 2 months of starting the therapy.
Treatment outcomes in patients with renal failure with sCr more than 1.3 mg/dl were similar among the three groups of patients (P = 0.44) [Table 2]. However, as compared to patients with normal renal function, lesser number of patients with reduced GFR had remissions. 8, 7, and 6 patients in NIH, ELNT, and MMF group required dialysis at presentation. Of these, 4 out of 8 in NIH group (50%), 3 of 7 in ELNT group (42.8%), and 3 of 6 in MMF group (50%) had remission. Mean SLEDAI in NIH, ELNT, and MMF group at 6 months was 10.1 ± 2.5, 11.3 ± 3.2, and 10.2 ± 2.2, respectively.
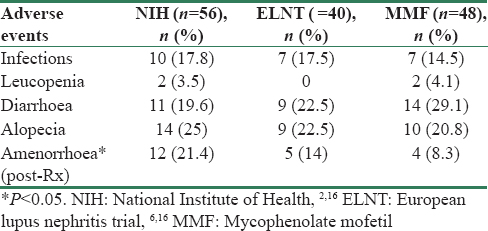
Adverse events comprised mainly of infections, leucopenia, diarrhea, alopecia, and menstrual abnormalities. Amenorrhea was seen in 12 (21.4%) in NIH group, 12 (21.4%) in ELNT group, and in 4 patients (8.3%) in MMF group.
In our study, 24 (16.6%) of the total patients had infections [Table 3]. Lower respiratory tract infection was seen in 8 patients, urinary tract infection in 5, tubercular pleural effusion, herpes zoster and chickenpox in 2 patients each, vaginal infection, cellulitis, paronychia, spontaneous bacterial peritonitis, and Aspergillus were seen in 1 each. The patient with Aspergillus (fungal aspergilloma) succumbed despite treatment. Four were viral infections while remaining all infections were bacterial. Although the infections were slightly higher with CPM (17.8% and 17.5% in NIH and ELNT) versus 14.5% in MMF group, the difference were not statistically significant. Steroid-related adverse reactions seen in our patients included diabetes in 2, corneal opacity in 4, cataract in 4, and avascular necrosis in 2 individuals. Other adverse events like deep vein thrombosis were seen in 3 individuals.
Among women in our study population, 64 had conceived before the disease, 4 had successful pregnancy posttreatment, 6 had abortions, and 10 females did not have children.
The cost of induction therapy in NIH arm was Rs. 750 (each injection vial of 500 mg costs around Rs. 75, and each person required approximately 5 g), for ELNT trial the cost of induction was Rs. 450, while in the MMF arm the induction cost was Rs. 16,200–21,600 (1.5–2 g/day for 6 months each 500 mg tablet costing around Rs. 35). As ours is a government hospital, the hospitalization for treatment of infections was free.
Discussion
In this study, we have compared NIH protocol (high-dose IV CPM protocol) with MMF and ELNT protocol (low-dose IV CPM protocol) as induction treatment for proliferative LN.[16] The treatment outcomes and adverse events in the three groups were studied.
Most of the studies in literature had a follow-up of 6 months (Appel et al.,[8] Ginzler et al.,[9] where the authors compared IV CPM and oral MMF). Chan et al. compared oral CPM with MMF and followed the patients for 1 year.[7] In the study by Houssiau et al. the follow-up period was of 10 years, where high-dose CPM and ELNT were compared.[6] In our study, we have compared the three treatment regimens NIH, ELNT, and MMF and studied outcomes at 6 months.
The mean dose of CPM in NIH protocol in Appel et al. study was 750 mg/m2 and the MMF dose used was 2–3 g/day; in ELNT trial by Houssiau et al., in high-dose CPM group the patients received 500 mg/m2 for 8 pulses (6 monthly and two quarterly pulses) and day 14 white blood cell (WBC) count was monitored and dose was increased until the nadir WBC count was 1500, with max of 1500 mg/pulse, in low dose group patients received 3 g over 6 doses. In our study, the dose of CPM for NIH group was 500 mg/m2/month for 6 doses followed by azathioprine; in ELNT group, 500 mg was given every 15 days for 6 doses followed by maintenance with azathioprine; in MMF group the dose was 1200 mg/m2 (1.5–2 g/day) for 6 months followed by azathioprine maintenance. Thus, in our study, we used a lower dose of CPM and MMF than Appel et al. study.
Ours was the youngest study population compared to others with patients having more severe renal failure than the patients in the other landmark studies though the proteinuria was comparable to the earlier studies. The proportion of renal failure was similar to Appel et al. group, in which 32 patients had GFR of <30 ml/min/m2, unlike the other studies who have excluded the patients with less GFR. Histology was similar with Class IV LN being the most common as in Houssiau study. Crescentic GN was seen in 12.5% in our patients, but in Houssiau et al. study 60%, had crescents as patients with focal crescents were also included in this group in their study.
Efficacy of various protocols
Complete remission - Appel et al. in their study demonstrated equivalent rates of remission in both the therapies [Table 4]. However, Ginzler et al. in their study showed the superiority of MMF over IV CPM, which was statistically significant. In Houssiau et al. study comparing high-dose CPM with ELNT, the remission rate was 54% and 71%, respectively, but follow-up period of the study was longer (73 m), their primary end points were doubling of creatinine, ESKD, and death. Remission was not a primary endpoint. In our study, complete and partial remission rates were not significantly different between the groups and were superior to those in the earlier studies. At the end of 6 months, the combined remission rate was 71.4%, 65%, and 72.9% in NIH, ELNT, and MMF groups, respectively. Ginzler et al. showed MMF was as effective in controlling nonrenal manifestations of lupus as CPM.
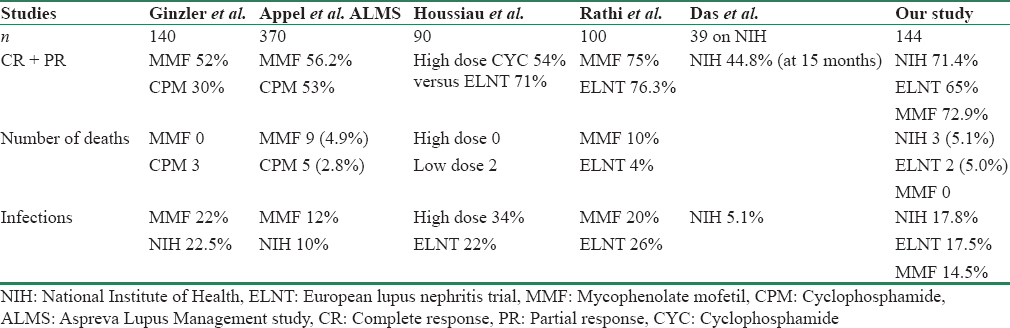
In our study, deaths were seen in 5 (3.5%) patients with the highest number in the NIH group. Death rates in our study were thus similar to the earlier studies.
Among the adverse events, the major adverse event was infection. In Houssiau et al. trial number of severe infections were 15, with more infections in the NIH group while in Appel et al. study infections were slightly higher in the MMF group while in the study by Ginzler et al. infections were comparable in MMF and NIH arms. Thus though CPM was thought to increase infection risk in some studies, we found it to be a safe drug for treatment of LN. Gonadal toxicity, in a study by Houssiau et al. was seen in 8 patients and was similar in ELNT and NIH, transient amenorrhea was seen in two individuals. In our study, among the different protocols, NIH was associated with greater percentage of amenorrhea as compared to the other two regimes.
Steroid-related adverse reactions were common and may have been due to the high dose of steroids used in our patients, which included methyl prednisolone during induction and subsequent oral steroids throughout the induction period.
In subgroup analysis, in the patients presenting with renal failure, there was no statistically significant difference in outcome between MMF and CPM, in our study as in Houssiau et al. study. However, other studies by Tang et al. demonstrated MMF is equal or superior to IV CPM in crescentic GN[17] Wang et al. demonstrated the efficacy of MMF in severe necrotizing vasculopathy due to lupus.[18]
The cost-effectiveness analysis done in the UK comparing MMF and NIH showed NIH is costlier than MMF, but they have included all the cost including the hospitalization cost for adverse events.[19] In our setting, the cost of the three treatment protocols was significantly different. Thus, NIH and ELNT were significantly associated with a lower cost as compared to MMF. IV CPM was given in a day care setting avoiding cost of hospitalization. Also as our hospital is a government hospital, there is no out of pocket payment required for nursing care, infusions, etc. In a private hospital setting, the difference in cost of therapy may be lesser.
In a meta-analysis by Radhakrishnan et al. analyzing geographical variation in the response of LN to MMF and CPM, MMF was found to be superior in people outside Asia like Hispanics and Africans, but the studies from Asia showed MMF was equivalent to IV CPM.[20] It has also shown that trials conducted in Asia had higher response rates compared to the other trials. A study from south India revealed that remission rates in a cohort of predominantly Class IV lupus patients were 74.3% and average time to remission was 15 months.[21] Rathi et al. compared MMF with low-dose CPM in the treatment of LN (Class III, IV, or V). However, unlike our study, those with crescentic LN, a high sCr and neurological or pulmonary lupus were excluded. MMF was prescribed at daily doses of 1.5–3 g for 24 weeks, while CPM was administered as six fortnightly infusions of 500 mg each. All patients received three methylprednisolone injections, followed by oral corticosteroids. The primary end point was treatment response at 24 weeks as in our study. Of the 173 patients recruited, 100 were equally randomized to receive either CPM or MMF i.e., 50 in each group. The complete remission rate was 50% in CPM and 54% in MMF group. Gastrointestinal symptoms were significantly more frequent in patients receiving MMF (52 vs. 4%). However, other adverse events were similar.[22] In our study, NIH, ELNT, and MMF were all found to be effective therapies with CPM-based therapies associated with a lower cost. CPM-based therapy had higher menstrual irregularities in our study [Table 4].
Drawbacks of the study
There were several limitations to our study. The study was done in a single center, and all patients were of the same ethnic group. The results may be different in other ethnic groups, and hence multicenter study may be needed for validation. The patients were on fixed dose of MMF in the MMF arm and concentration of MMF were not monitored, hence, the levels may have been inadequate is some and high in others depending on the varying pharmacokinetics in these patients. Our study also had a short follow-up.
Conclusions
MMF- and CPM-based regimens are equally effective for the treatment of LN. CPM-based regimes provide a cheaper alternative in resource-limited settings. Low-dose CPM regime may provide equal efficacy with further reduction of cost and drug toxicity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Systemic lupus erythematosis. In: Harrison T, Kasper D, Fauci A, Hauser S, Longo D, Jameson J, eds. Harrison's Principles of Internal Medicine (19th ed). New York: McGraw-Hill Education; 2015. p. :2124-34.
- [Google Scholar]
- Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614-9.
- [Google Scholar]
- Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N Engl J Med. 1978;299:1151-5.
- [Google Scholar]
- Controlled trial of pulse methyl prednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet. 1992;340:741-5.
- [Google Scholar]
- Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549-57.
- [Google Scholar]
- Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-31.
- [Google Scholar]
- Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343:1156-62.
- [Google Scholar]
- Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103-12.
- [Google Scholar]
- Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219-28.
- [Google Scholar]
- American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64:797-808.
- [Google Scholar]
- Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771-82.
- [Google Scholar]
- KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2(Suppl 2):S139-274.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521-30.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241-50.
- [Google Scholar]
- Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630-40.
- [Google Scholar]
- Induction and maintenance treatment of proliferative lupus nephritis: A meta-analysis of randomized controlled trials. Am J Kidney Dis. 2013;61:74-87.
- [Google Scholar]
- Effects of mycophenolate mofetil for patients with crescentic lupus nephritis. Nephrology (Carlton). 2008;13:702-7.
- [Google Scholar]
- Induction therapies for class IV lupus nephritis with non-inflammatory necrotizing vasculopathy: Mycophenolate mofetil or intravenous cyclophosphamide. Lupus. 2007;16:707-12.
- [Google Scholar]
- The cost-effectiveness of mycophenolate mofetil as firstline therapy in active lupus nephritis. Rheumatology (Oxford). 2007;46:1096-101.
- [Google Scholar]
- Geographical variation in the response of lupus nephritis to mycophenolate mofetil induction therapy. Clin Nephrol. 2011;75:233-41.
- [Google Scholar]
- The outcome of proliferative lupus nephritis with pulse cyclophosphamide therapy. Indian J Nephrol. 2011;21:160-5.
- [Google Scholar]
- Comparison of low-dose intravenous cyclophosphamide with oral mycophenolate mofetil in the treatment of lupus nephritis. Kidney Int. 2016;89:235-42.
- [Google Scholar]







