Translate this page into:
Nasal Methicillin Resistant Staphylococcus Aureus Colonisation and the Incidence of Invasive Staphylococcal Infection in Patients Undergoing Hemodialysis
Corresponding author: Lisha Pallivalappil, Department of Pulmonary Medicine and Critical Care, Amala Institute of Medical Sciences, Thrissur, Kerala, India. E-mail: drlishapvranjith@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mathew N, Suresh SA, Pallivappil L, Suseela KV. Nasal Methicillin Resistant Staphylococcus Aureus Colonisation and the Incidence of Invasive Staphylococcal Infection in Patients Undergoing Hemodialysis. Indian J Nephrol. 2025;35:419-21. doi: 10.25259/IJN_418_2024
Dear Editor,
Methicillin resistant Staphylococcus aureus (MRSA) is a nosocomial pathogen associated with significant morbidity and mortality. People with diabetes, intravenous drug abusers, and patients with recurrent hospital admissions are more likely to develop infection, usually following colonization. This makes targeted screening an attractive option for infection prevention and control.1,2 Data on MRSA surveillance and invasive infections in rural to semi-urban settings of low- and middle-income countries are essential for formulating infection surveillance strategies and antibiotic policies. The current study aims to determine the MRSA colonization prevalence in a cohort of patients with end-stage renal disease (ESRD) undergoing dialysis.
This prospective observational cohort study was done in the hemodialysis (HD) unit in Kerala. The hospital predominantly caters to middle income patients. Demographic data, and previous hospitalization details, including infection with Staphylococcus were collected from hospital records. Comorbid illnesses were defined according to standard criteria.
Trained personnel inserted dry swabs 1 cm into each nasal vestibule and rotated 4 times while maintaining even contact with the nasal mucosa. All specimens were collected within 12 hours of admission. Culture and sensitivity tests were done according to standard methodology.3 Those positive for MRSA colonization were treated with mupirocin topical ointment and retested every 3 weeks until negative.
Patients were followed up every three months for 1 year. Infections were defined according to National Healthcare Safety Network (NHSN) surveillance definitions.4
Colonization was defined as isolating MRSA from nasal smears without evidence of active infection at any site. Invasive infections were determined according to Centre for disease control and prevention NHSN surveillance criteria.4
A total of 123 patients were included in the study. Two patients did not consent and two were lost to follow up. The final analysis included 119 patients. The demographic data is shown in Table 1. Males were predominant, and the majority were undergoing HD via arteriovenous fistula 2-3 times a week. Eight patients had internal jugular or femoral dialysis catheters. Four patients were positive for MRSA. All four underwent nasal decolonization with mupirocin ointment topical application for five days and when subjected to repeat swabbing after two weeks, were found to be negative. On follow-up, 25 (21%) patients had developed invasive infections. Eight patients had staphylococcal infections, of which two were bloodstream, and six were skin and soft tissue infections. None of these patients had positive surveillance nasal swabbing. The predominant infections were bloodstream, skin, and soft tissue infections. The distribution of isolated organisms is given in Figure 1. Pseudomonas aeruginosa was the most frequently isolated organism.
| Number (%) | |
|---|---|
| Gender | |
| Male | 97 (80) |
| Female | 24 (20) |
| Comorbidities | |
| Diabetes | 70 (57.9) |
| Hypertension | 38 (31.4) |
| CAD | 110 (90.9) |
| CVA | 24 (19.8) |
| Frequency of hemodialysis | |
| Once weekly | 3 (2.5) |
| Twice weekly | 31 (25.6) |
| Thrice weekly | 87 (71.9) |
| Duration of hemodialysis | |
| Upto three months | 11 (9.1) |
| 3 months to 1 year | 21 (17.3) |
| More than 1 year | 89 (73.6) |
| Hospital admission in the last 90 days | 32 (26.4) |
| Risk factors for MRSA colonization | |
| Skin and soft tissue infections | 9 (7.4) |
| Presence of implants or devices | 12 (9.9) |
| Intravenous antibiotics in the last 90 days | 44 (36) |
| Recent surgery or interventional procedure | 118 (97.5) |
| Comorbid illnesses | 116 (96) |
| Smoking | 13 (10.7) |
| Presence of AV fistula | 111 (91.7) |
CAD: Coronary artery disease, CVA: Cerebrovascular accident, MRSA: Methicillin resistant Staphylococcus aureus, AV: Arteriovenous.
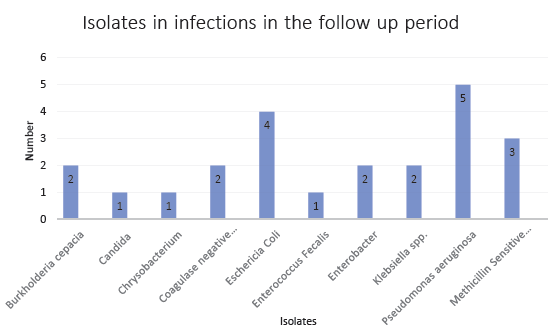
- Isolation of pathogens in invasive infections in the follow up period. MSSA: Methicillin-sensitive Staphylococcus aureus.
Nasal colonization with MRSA is considered a risk factor for invasive infections. Invasive infections by MRSA are associated with high mortality in ESRD patients.5 The human nose is the largest ecological reservoir for human strains of Staphylococcus aureus. Around the world, the incidence of MRSA nasal carriage has ranged from 2% to 45%.6
The MRSA colonization data on Indian patients range from 10% to 80%. The pooled prevalence of MRSA in India from 2015 to 2020 was 37%.7,8 One study on nasal carriage by healthcare workers in an ICU in Kerala found an 18% carrier rate among ICU staff.9 Carriage rates in community settings have been reported to be lower. The variation in the MRSA nasal carriage rate in various studies can be attributed to the admission rates, the prevalent infection prevention measures, and whether the study was done during an outbreak.
The 3% detection rate in this study is low compared to other similar studies.10,11 The nasal swabbing, done within 12 hours of admission to the hospital for dialysis, could mean that our study population effectively represented the relatively low rates in the community.
The other important observation was that the major pathogens causing invasive infections in the follow-up period belonged to the gram-negative group. Pseudomonas aeruginosa had the greatest number of isolations from blood and pus samples. This questions the usefulness of nasal surveillance and MRSA colonization detection and eradication programs in preventing infections in patients undergoing HD in our setting. Whether the surveillance target should move towards gram-negative infections rather than gram-positive ones is a question to be answered by further research.
Conflicts of interest
There are no conflicts of interest.
References
- Methicillin-resistant staphylococcus aureus: An overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing (30th ed). Clinical and Laboratory Standards Institute; 2023. CLSI document M100
- The National Healthcare Safety Network (NHSN) Manual: NHSN [2022] Toolkit and Guidance for External Validation. Atlanta, GA: Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; https://www.cdc.gov/nhsn/pdfs/validation/2022/2022-nhsn-ev-guidance-508.pdf
- Innovative therapies for acute bacterial skin and skin-structure infections (ABSSSI) caused by methicillin-resistant Staphylococcus aureus: Advances in phase I and II trials. Expert Opin Investig Drugs. 2020;29:495-506.
- [CrossRef] [PubMed] [Google Scholar]
- Nasal carriage rate of staphylococcus aureus, Its associated factors, and antimicrobial susceptibility pattern among health care workers in Public Hospitals, Harar, Eastern Ethiopia. Infect Drug Resist. 2023;16:3477-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of methicillin-resistant Staphylococcus aureus in India: A systematic review and meta-analysis. Oman Med J. 2022;37:e440.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A systemic literature review and meta-analysis reporting the prevalence and impact of methicillin-resistant staphylococcus aureus infection in India. Infect Dis (Auckl). 2020;13:1178633720970569.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nasal carriage of meticillin-resistant Staphylococcus aureus amongst healthcare workers of intensive care unit of a tertiary care centre in South India: An observational cross-sectional study. J Patient Saf Infect Control. 2020;8:64-7. Available from: https://www.jpsiconline.com/text.asp?2020/8/2/64/304219
- [Google Scholar]
- Methicillin-resistant staphylococcus aureus in intensive care unit setting of India: A review of clinical burden, patterns of prevalence, preventive measures, and future strategies. Indian J Crit Care Med. 2020;24:55-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patterns of microbial resistance in bloodstream infections of hemodialysis patients: A cross-sectional study from Palestine. Sci Rep. 2022;12:18003.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







