Translate this page into:
Nephrology’s Next Frontier: Expanding the Reach of CAR T-Cell Therapy for Refractory Lupus Nephritis and Beyond
Corresponding author: Narayan Prasad, Department of Nephrology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India. E-mail: narayan.nephro@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Meyyappan J, Prasad N. Nephrology’s Next Frontier: Expanding the Reach of CAR T-Cell Therapy for Refractory Lupus Nephritis and Beyond. Indian J Nephrol. doi: 10.25259/IJN_274_2024
Abstract
Chimeric antigen receptor (CAR) T-cell therapy has recently evolved beyond cancer therapy’s boundary to treating autoimmune diseases such as lupus nephritis. In CAR T-cell therapy, the genetically engineered patient’s T cells express a receptor specifically targeting antigens such as CD19, a protein found on the surface of B cells. By directing the immune system to eliminate B cells, which play a central role in the pathogenesis of systemic lupus erythematosus, CAR T-cell therapy offers a novel and potent approach to resetting the immune system and achieving remission in difficult-to-treat lupus nephritis patients and many such conditions in nephrologists’ practice.
Keywords
Chimeric antigen receptor T-cell
Cancer
Lupus nephritis
Autoimmune diseases
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is increasingly utilized for numerous refractory autoimmune diseases, including systemic lupus erythematosus (SLE), which exhibits an estimated prevalence of 30–1590 per million population in India.1 This chronic, multisystem autoimmune disease is characterized by immune complex-mediated damage to various organs, most notably the kidneys. Lupus nephritis (LN) affects 60–80% of patients, leading to higher morbidity and mortality.2 It frequently manifests early, within the first 5 years of diagnosis. Juvenile-onset SLE, typically diagnosed at approximately 12.6 years of age, is associated with higher disease activity and more severe outcomes compared to adult-onset SLE.3 These young patients face a poor prognosis with severe organ damage, diminished quality of life, and the potential necessity for kidney replacement therapy. The standard treatment regimen, which combines corticosteroids with immunosuppressants, has improved survival rates of LN patients, increasing from a 17% survival rate at 5 years in untreated patients to 80%. The commonly utilized regimens include Mycophenolate Mofetil (MMF), Cyclophosphamide, Belimumab, Rituximab, and Calcineurin inhibitors, including the novel agent Voclosporin.4 These regimens, however, are not without adverse effects. Refractory LN, variably defined as the absence of any remission with cyclophosphamide-based NIH regimen and standard doses of MMF, may occur in 20 to 70% of patients.5 Approximately 10–30% of LN patients still progress to ESRD within 15 years.6 For these difficult-to-treat LN patients, innovations in management utilizing CAR T-cell therapy show promise.6
Evolution of chimeric antigen receptor T cell therapy
Wayne A. Marasco conceptualized the development of T cell-based therapy for cancer after he developed developed renal cell carcinoma (RCC). The CD19-targeted CAR T-cell therapy is considered fourth-generation therapy for RCC.7 Cytotoxic T-cells, also called killer T-cells, kill cancer cells directly by finding cancer cells and can be stimulated to kill cancer cells. Helper T-cells fight cancer indirectly by organizing and orchestrating the fight against cancer cells. Autologous lymphocytes from the patients are genetically engineered ex vivo to make them coexpress an antibody specific for a tumor/lymphocyte-associated antigen with the help of a viral vector with CAR DNA and an activation signal triggering the production of cytotoxic factors, which kills the target cells. The principle of engineering CAR T cells and therapy is depicted in Figure 1.8,9
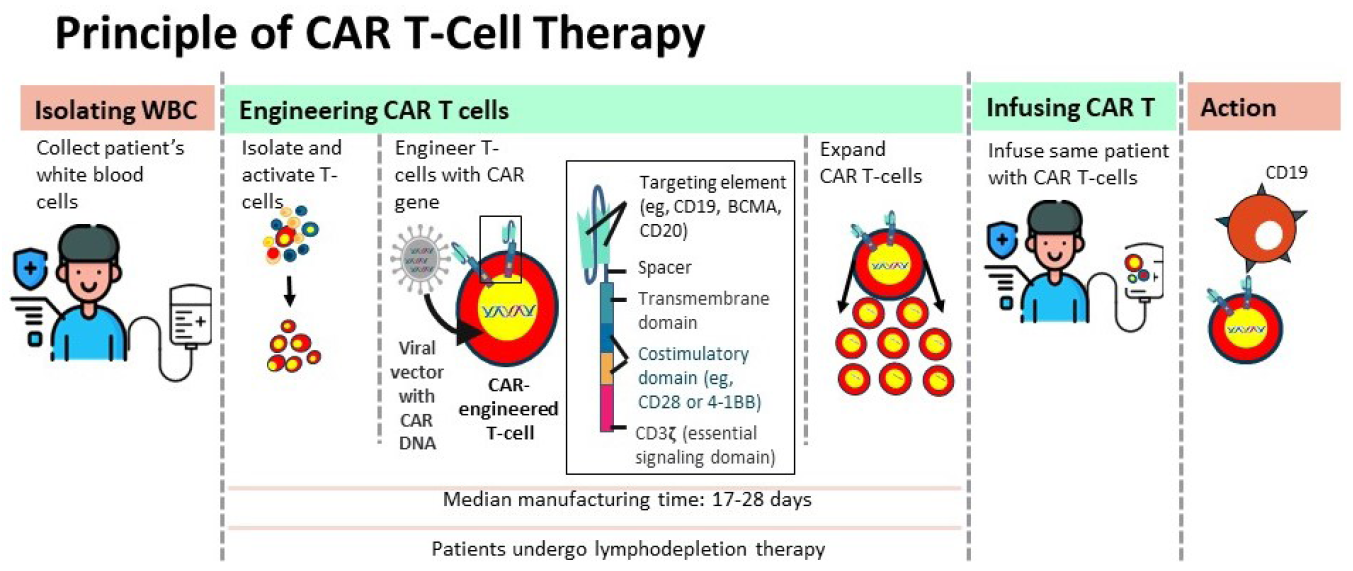
- Infographic of the principle of chimeric antigen receptor (CAR) T-cell therapy.
The evolution of CARs has progressed through five generations, starting with the first-generation CARs containing only a CD3ζ intracellular domain, which showed limited cytotoxicity and proliferation. Second-generation CARs introduced costimulatory domains like CD28 or CD137 to enhance T-cell activity, while third-generation CARs added a second costimulatory domain (e.g., CD134). Fourth-generation CARs, or TRUCKs, included inducible cytokines such as IL-12 for enhanced tumor killing. Fifth-generation CARs incorporate a truncated IL-2Rβ domain that activates JAK–STAT signaling, offering full T cell activation through synergistic TCR, costimulatory, and cytokine signals. In patients with relapsed or refractory multiple myeloma, the B-cell maturation antigen (BCMA) is emerging as a promising candidate antigen for CAR-T therapy. Other antigens being explored for the target include CD38, CD138, SLAMF7, CS1, and GPRC5D. In addition to T cells, natural killer cells (CAR-NK) can be engineered and customized against tumor antigens, especially those of solid tumors. Exploration experiments are currently ongoing for the use of CAR-NK cell therapy in patients with RCC, lung, breast, and pancreatic cancers, and glioblastoma.10
In 2017, FDA approved the first CAR T-cell therapy - Tisagenlecleucel - for B-cell malignancies. To date, six second-generation CAR T-cell therapies targeting CD19 and BCMA have been licensed for treating aggressive hematological malignancies, including relapsed-refractory (R/R) B-cell lymphoma and multiple myeloma.
CAR T-cell therapy for autoimmune diseases
CAR T cell therapy has emerged as a promising treatment modality for various autoimmune diseases, including SLE. In the context of SLE, this approach involves the genetic modification of a patient’s T cells to express a receptor specifically targeting CD19, a protein expressed on the surface of B cells. By directing the immune system to eliminate B cells, which play a central role in the pathogenesis of SLE, CAR T-cell therapy presents a novel and potentially efficacious approach to resetting the immune system and inducing remission. A 2021 proof-of-concept study demonstrated that one patient with refractory SLE achieved serologic and clinical remission within 7 weeks of a single infusion. Subsequent research in 2022 yielded similar results in five patients, all of whom entered remission within 3 months and remained drug-free for a median of 8 months.11 A study involving 15 patients with SLE, idiopathic inflammatory myositis, and systemic sclerosis demonstrated sustained efficacy of CAR-T therapy up to 2 years, with a median follow-up of 15 months, assessed by DORIS remission criteria and other disease-specific measures.12 A recent case from University Hospital Erlangen, Germany, exemplifies the transformative potential of CAR T-cell therapy for severe LN.13 A 15-year-old girl with rapidly progressive SLE experienced severe kidney involvement despite exhaustive conventional treatments, including hydroxychloroquine, azathioprine, mycophenolate mofetil, and the B-cell-targeting antibody belimumab. Her condition deteriorated rapidly, with proteinuria exceeding 10,000 mg/g creatinine and creatinine levels rising to 4.96 mg/dL. In this dire situation, clinicians turned to CD19-targeted CAR T-cell therapy, administered under a compassionate use protocol. This involved harvesting the patient’s T cells, genetically modifying them to target CD19, and reinfusing them after a tailored lymphodepletion regimen. The patient’s kidney function began to improve within days, and she was dialysis-free by the end of the treatment course. Creatinine levels fell to 1.2 mg/dL, and proteinuria decreased. Additionally, the patient could taper off most of her medications and return to school, enjoying a quality of life that had seemed impossible just months earlier.
This case underscores the transformative potential of CAR T-cell therapy in treating severe autoimmune diseases like SLE. It not only halted the progression of the disease but reversed the severe kidney damage, and highlights the importance of early and aggressive intervention, particularly in juvenile-onset SLE, where rapid disease progression can lead to irreversible organ damage within a short span.
One of the key advantages of CAR T-cell therapy is its targeted approach. By focusing on CD19, a marker present on B cells, the therapy specifically eliminates the cells responsible for producing autoantibodies that drive the pathological processes in SLE. This precision reduces the likelihood of off-target effects. It provides a more sustainable remission compared to broad-spectrum immunosuppressive therapies, which can leave patients vulnerable to infections and other complications.14 The success of CAR T-cell therapy also highlights the importance of personalized medicine. Each patient’s T cells are engineered to target their specific disease markers, offering a customized treatment more likely to succeed where conventional therapies have failed. This approach represents a significant shift in treating other autoimmune diseases, moving from one-size-fits-all solutions to more individualized interventions.
The medical community must now focus on further research and clinical trials to validate and expand this promising therapeutic option. Ensuring that more young patients have access to CAR T-cell therapy could transform the outlook for many grappling with devastating diagnoses like LN.
The economic implications of successful CAR T-cell therapy cannot be ignored. The cost of treatment is likely to be extremely high, out of reach of a vast majority of populations in the law and middle income countries. A case can be made that even though the upfront costs are high, the long-term savings from reduced healthcare needs, such as fewer hospitalizations and the elimination of lifelong dialysis, can be substantial. This economic argument is particularly compelling in severe autoimmune diseases, where traditional treatments often incur ongoing costs and significant healthcare resource utilization. Countries and jurisdictions with multiple competing challenges however must make decisions on the basis of appropriate health technology assessment.
Challenges, solutions and Adverse effects of CAR T-cell therapy
While this success story is promising, it also calls for cautious optimism. CAR T-cell therapy’s long-term safety and efficacy in pediatric patients need thorough evaluation through clinical trials. The experience of this single patient, although encouraging, must be validated in larger cohorts. The most common side effects include cytokine release syndrome (CRS), occurring in more than 80% of patients, and neurotoxicity syndrome, in 64% of patients. Renal and electrolyte abnormalities are also expected, including acute kidney injury (19%), hyponatremia (75%), hypokalemia (56%), and hypophosphatemia (51%) and macrophage activation syndrome.15 In a study by Wood et al., among 166 CAR T-cell-treated patients, 23% experienced acute kidney injury (AKI), with 87% occurring after CAR T-cell infusion. Most cases were Grade 1 (69%), while Grade 2 (13%) and 3 (18%) AKI were also observed. The most common cause was decreased renal perfusion (72%), with cytokine release syndrome (CRS) contributing to 44% of AKI cases.16 Careful monitoring and appropriate management of CRS may involve supportive care with or without tocilizumab and high-dose steroids.
Future potential in the management of other kidney diseases
The potential of CAR T-cell therapy extends beyond lupus nephritis and holds promising for other kidney diseases with immune dysregulation. For example, CAR T-cell therapy could potentially treat primary membranous nephropathy and IgA nephropathy and other glomerular diseases with autoimmune etiopathogenesis. By specifically depleting the B cells responsible for the pathological autoantibodies, CAR T-cell therapy could provide a new therapeutic avenue, offering hope to patients who have not responded to conventional treatments.
CAR T-cell therapy is emerging for use in desensitization in sensitized kidney transplantation. A recent study by Jarmi et al. at the Mayo Clinic validated this concept by enlisting 10 highly sensitized patients.17 Jarmi et al.17 successfully demonstrated CAR T-cells’ antigen-specific functionality by targeting B-cell activating factor receptor (BAFF-R)-positive and BAFF-R-negative Nalm-6 cells. The experimental agents MC10029 CAR T cells derived from sensitized patients exhibited cytotoxic effects against autologous B cells, which substantiates the viability of employing B-cell–targeting CAR T-cell therapy as a desensitization strategy. Its use in refractory post-transplant lymphoproliferative disorder is another promising field of CAR T-cell therapy.18,19
Despite these promising advancements, CAR-cell therapy’s current scope in kidney-related diseases is confined to just four conditions: RCC, relapsed or refractory multiple myeloma, SLE – Lupus Nephritis refractory to standard of care, and HIV-AIDS,20 and for desensitization in highly sensitized patients.12 This limitation underscores the necessity for continued research to expand its applications to encompass a broader range of kidney diseases.
Conflicts of interest
There are no conflicts of interest.
References
- Global and regional prevalence and incidence of systemic lupus erythematosus in low-and-middle income countries: A systematic review and meta-analysis. Rheumatol Int. 2022;42:2097-107.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24:1357-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Differences in disease phenotype and severity in SLE across age groups. Lupus. 2016;25:1542-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO 2024 clinical practice guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024;105:S1-S69.
- [CrossRef] [PubMed] [Google Scholar]
- Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219-28.
- [CrossRef] [PubMed] [Google Scholar]
- Current and emerging therapies for lupus nephritis. J Am Soc Nephrol. 2016;27:2929-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chimeric antigen receptor T cells therapy in solid tumors. Clin Transl Oncol. 2023;25:2279-96.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35-45.
- [CrossRef] [PubMed] [Google Scholar]
- The principles of engineering immune cells to treat cancer. Cell. 2017;168:724-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Breakthrough of solid tumor treatment: CAR-NK immunotherapy. Cell Death Discov. 2024;10:40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567-9.
- [CrossRef] [PubMed] [Google Scholar]
- CD19 CAR T-cell therapy in autoimmune disease - A case series with follow-up. N Engl J Med. 2024;390:687-700.
- [CrossRef] [PubMed] [Google Scholar]
- CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. Lancet. 2024;403:1627-30.
- [CrossRef] [PubMed] [Google Scholar]
- CAR T-cell therapy: Adverse events and management. J Adv Pract Oncol.. 2019;10(Suppl 3):21-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis. 2020;76:63-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outcomes of CD19-targeted chimeric antigen receptor T cell therapy for patients with reduced renal function including dialysis. Transplant Cell Ther. 2022;28:829.e1-829.e8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CAR T-cell therapy-paving the way for sensitized kidney transplant patients. Kidney Int. 2024;105:1124-9.
- [CrossRef] [PubMed] [Google Scholar]
- Successful CAR T cell therapy in a heart and kidney transplant recipient with refractory PTLD. JACC CardioOncol. 2022;4:713-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CAR T-cell therapy for refractory posttransplantation lymphoproliferative disorder in a kidney transplant patient. Transplant Direct. 2024;10:e1584.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CAR-T cell therapy: Advances in kidney-related diseases. Kidney Dis (Basel). 2024;10:143-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







