Translate this page into:
Neutrophil-to-lymphocyte ratio, insulin resistance, and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease
Address for correspondence: Dr. Kultigin Turkmen, Department of Nephrology, Selcuk University Meram School of Medicine Konya, Turkey. E-mail: mdkt2010@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Endothelial dysfunction (ED), insulin resistance (IR), and inflammation are risk factors for increased cardiovascular morbidity and mortality in autosomal dominant polycystic kidney disease (ADPKD). ADPKD patients may have increased carotid intima-media thickness (CIMT) and decreased coronary flow velocity reserve (CFVR). The neutrophil-to-lymphocyte ratio (NLR) was introduced as a marker to determine inflammation in various disorders. We aimed to investigate the relationship between NLR and IR, CFVR, CIMT, and the left ventricular mass index (LVMI) in normotensive ADPKD patients. Twentynine ADPKD patients (age 38.8 ± 10.2 years; 8 men and 21 women) and 19 healthy controls (age 33.8 ± 7.4 years; 8 men and 11 women) were included in this cross-sectional study. CFVR was calculated with echocardiography as the ratio of hyperemic to baseline diastolic peak coronary flow velocities. CIMT was measured in the distal common carotid artery by using a 10-MHz linear echocardiography probe. HOMA-IR was calculated NLR was calculated as the ratio of the neutrophil and lymphocyte counts. Age, sex, body mass index, and levels of glucose, creatinine, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, C-reactive protein (CRP), microalbuminuria, and creatinine clearance were similar between ADPKD patients and healthy subjects. NLR, CIMT, LVMI, and HOMA-IR were significantly higher and CFVR values were significantly lower in patients with ADPKD compared to that in healthy subjects. NLR showed positive correlation with CIMT, HOMA, insulin, glucose, and HDL cholesterol levels, while it was inversely correlated with CFVR and albumin level in all subjects. In patients with ADPKD, NLR showed positive correlation with HDL cholesterol level and inverse correlation with LVMI and albumin level. NLR that was found to be increased in patients with ADPKD may be a readily available marker of inflammation and ED.
Keywords
Autosomal dominant polycystic kidney disease
endothelial dysfunction
insulin resistance
neutrophil-to-lymphocyte ratio
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is associated with high cardiovascular (CV) morbidity and mortality.[1] Endothelial dysfunction (ED), hypertension, left ventricular hypertrophy (LVH), diastolic dysfunction, and insulin resistance (IR) are the most commonly encountered risk factors in the pathogenesis of these increased CV events in patients with ADPKD.[2–5] An association between IR and LVH was found in these patients, independent of other factors known to increase the left ventricular mass index (LVMI).[6] Recent studies showed that early interventions can reverse increased CV events because the risk factors mentioned earlier mostly occur in the early course of ADPKD before the onset of chronic kidney disease in this population.[7–9]
Coronary flow velocity reserve (CFVR), evaluated by noninvasive echocardiography, represents the capacity of coronary circulation to dilate after dipyridamole infusion. By using this method, impairment of CFVR can be assessed before the development of angiographically detectable stenosis in epicardial coronary arteries. In our previous study, we demonstrated that normotensive ADPKD patients with well-preserved renal function had significantly increased carotid intima-media thickness (CIMT) and significantly decreased CFVR when compared with healthy subjects, which means that atherosclerosis plays an important role in the early phases of cardiovascular injury seen in the pathogenesis of ADPKD.[2]
Inflammation was also demonstrated in young ADPKD patients with preserved kidney function.[1011] Merta et al.[12] showed that ADPKD patients had higher plasma levels of interleukin (IL)-6 and IL-8 as markers of inflammation. In a cohort of patients at different stages of ADPKD, Menon et al.[10] demonstrated that levels of inflammatory markers were highest in patients with the worst kidney function.
In recent years, neutrophilia and relative lymphopenia were shown to be an independent predictor of mortality in patients with acute heart failure.[1314] Moreover, neutrophil-to-lymphocyte ratio (NLR) was introduced as a potential marker to determine inflammation in cardiac and noncardiac disorders.[15–17] As neutrophil and lymphocyte values are readily available in routine blood count analysis, NLR may be used as a cost-effective predictor of inflammation and cardiovascular complications.
Despite our knowledge about increased inflammation in ADPKD, the data about NLR and its association with inflammation and ED are lacking in this population. Therefore, we aimed to investigate the relationship between NLR and IR, CFVR, CIMT, and LVMI in normotensive ADPKD patients with preserved renal function.
Materials and Methods
The study protocol was approved by the Medical Ethics Committee of Istanbul University (Istanbul School of Medicine, Istanbul, Turkey). Written informed consent was obtained from all subjects included in the study.
Twenty-nine normotensive ADPKD patients (8 men and 21 women) with a mean age of 38.8 ± 10.2 years and 19 healthy controls (8 men and 11 women) with a mean age of 33.8± 7.4 years were included in this cross-sectional study. Healthy subjects and ADPKD patients were selected randomly from the Division of Nephrology of Istanbul University School of Medicine, Istanbul, Turkey. The diagnosis of ADPKD was defined by the ultrasonographic criteria described by Ravine et al.,[18] and all patients had positive family history of ADPKD. Patients aged 18-70 years willing to participate in the assessment of CFVR and CIMT by echocardiography were screened. A review of medical records was undertaken.
ADPKD patients with diabetes mellitus, established cardiovascular disease, chronic diseases that could affect endothelial function, thyroid or liver function abnormalities, a creatinine clearance of lower than 60 mL/min/1.73 m2, or a family history of premature atherosclerosis were excluded.
Among a total of 46 ADPKD patients screened, four had established cardiovascular disease, five had diabetes, five had creatinine clearance lower than 60 mL/min/1.73 m2, and three had liver function abnormalities. The remaining 29 ADPKD patients fulfilled the above criteria and were enrolled in the study. Nineteen age-matched and sex-matched healthy individuals referred from outpatient clinics of the Internal Medicine Department of Istanbul University were also enrolled as control subjects. They were subject to the same inclusion and exclusion criteria as the patients. Six patients with ADPKD and six healthy subjects were smokers. None of the subjects had a history of ethanol intake. Four patients with ADPKD and two healthy subjects were postmenopausal women. None of the subjects received any medications.
Systolic and diastolic blood pressures were measured on the right arm of subjects in an upright sitting position after at least 5 min of rest using an Erka sphygmomanometer (PMS Instruments Ltd, Berkshire, UK) with the appropriate cuff size. Two readings were recorded for each individual. The average of the two readings was defined as the subject's blood pressure.
Ambulatory blood pressure monitoring (ABPM) was performed in all patients over a 24 h period by the oscillometric method using a fully automatic noninvasive recorder (Spacelabs 90207, Redmond, Washington, USA). The monitor was programmed to measure blood pressure and heart rate at 20-min intervals between 07.00 and 22.59 and at 30-min intervals between 23.00 and 06.59. The patients were advised to pursue their usual daily activities; none was on nightshift duty, and they all slept during the night. All data were transferred into a software program.
Blood samples were drawn after an overnight fast of 12 h. All biochemical analyses including glucose, creatinine, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride concentrations were performed with an oxidase-based technique using Roche/Hitachi Modular System (Japan) in our central biochemistry laboratory. Fasting plasma insulin levels were determined from blood samples stored at –70°C by means of a commercial double-antibody solid-phase radioimmunoassay (Phadeseph Insulin RIA 100; Pharmacia Diagnostics AB, Uppsala, Sweden). Creatinine clearances were calculated by the Cockcroft–Gault formula.[19] Complete blood counts with automated differential counts, which included total white blood cells, neutrophils, and lymphocytes, were obtained at the time of admission. NLR was calculated as the ratio of the neutrophil and lymphocyte counts. The homeostasis model of IR (HOMA-IR) was used as a measure of IR.[20] HOMA-IR was calculated with the following formula: Fasting plasma glucose (mg/dL) times fasting serum insulin (∝IU/mL) divided by 22.5.
Echocardiographic examinations
Echocardiographic examination was performed using a Vingmed System Five, Norway echocardiographic system equipped with 2.5-MHz transducers (Vingmed Sound, Norway). M-mode and two-dimensional measurements were performed in accordance with the methods recommended by the American Society of Echocardiography.[2122] Cardiac mass was calculated by means of the formula derived by Devereux and Reichek.[23]
Carotid intima-media thickness measurements
The carotid arteries were evaluated with the Vivid 7 echocardiography device (General Electrics, USA) by using a 10-MHz linear probe. The acquired images were recorded for playback analysis and were later measured off-line. The common carotid artery, the carotid bulb, and internal and external carotid arteries were visualized on both sides. The intima media thickness (IMT) was measured in the distal common carotid artery at a level 15-20 mm proximal to the carotid bulb. The two bright echogenic lines in the arterial wall were identified as the intima and the media. Three measurements were made for each side of the body; separate means were calculated and recorded as the right and left IMT. None of the patients had stenosis, atheroma plaque, or local thickening in excess of 2 mm in the carotid arteries. Two different operators measured the CIMT, and the intraobserver coefficient of variation for CIMT was 2%.
Coronary flow velocity measurements
The CFV recordings were performed by a single investigator (HO) who was blinded for the two groups. CFV recordings were performed with the Vivid 7 echocardiography device (General Electrics, USA) using a middle range frequency (3-8 MHz) broadband transducer. CFV recordings were performed in the left anterior descending (LAD) coronary artery by transthoracic Doppler echocardiography (TTDE), as previously described.[24] The acoustic window was around the midclavicular line in the fourth and fifth intercostal spaces in the left lateral decubitus position. The left ventricle was imaged in the long-axis cross-section and the ultrasound beam was inclined laterally. The coronary blood in the mid-to-distal LAD artery was searched by color Doppler flow mapping guidance with the optimal velocity range (+12 to +15 cm/s). Then, the sample volume (1.5 or 2 mm wide) was positioned on the color signal in the LAD artery. Variables of LAD artery velocity were measured using fast Fourier transformation analysis. After baseline recordings of flows, dipyridamole (Persantin, Boehringer Ingelheim, 0.56 mg/kg) was infused over a 4-min period. An additional infusion of dipyridamole (0.28 mg/kg over a 2-min period) was used if the heart rate did not exceed a 10% increase from the baseline. One patient with ADPKD and one subject in the healthy control group needed a second dose of dipyridamole injection. Two minutes after the end of the infusion, hyperemic spectral profiles in the LAD artery were recorded. All images were recorded for playback analysis and were later measured off-line. Average peak diastolic velocity (APDV) was measured at baseline and under hyperemic conditions. The number of cardiac cycles from which the average APDV was derived was three, and the variation in APDV between each cycle was lower than 3%. CFVR was defined as the ratio of APDV at hyperemia to APDV at baseline. The intraobserver variability of CFVR measurement was 3.1% in this study.
All of the measurements were performed between 8:00 and 9:00 am, and all the subjects abstained from smoking and caffeine-containing drinks for at least 12 h before testing.
Statistical analyses
Statistical analyses were carried out using the Statistical Package for Social Sciences for Windows version 15.0 (SPSS, Chicago, IL, USA). Descriptive data are expressed as mean ± SD. Differences were considered significant when P values were less than 0.05. The strength of correlation coefficient (r) was defined as 0.2-0.4, weak; 0.4-0.7, moderate; and >0.7, strong. The normality of distribution of all variables was tested using the Kolmogorov–Smirnov test. Dichotomous variables were compared using the Chi-square test. Statistical differences between parametric data of two groups were analyzed using the Student's t-test. The Mann–Whitney U-test was used to compare nonparametric data. Linear associations between continuous parametric variables were assessed using the Pearson correlation test, while the Spearman correlation test was used to assess the correlation between nonparametric continuous and categorical variables. Association between NLR and CFVR was controlled for the other related variables using linear regressions with the backward Wald method.
Results
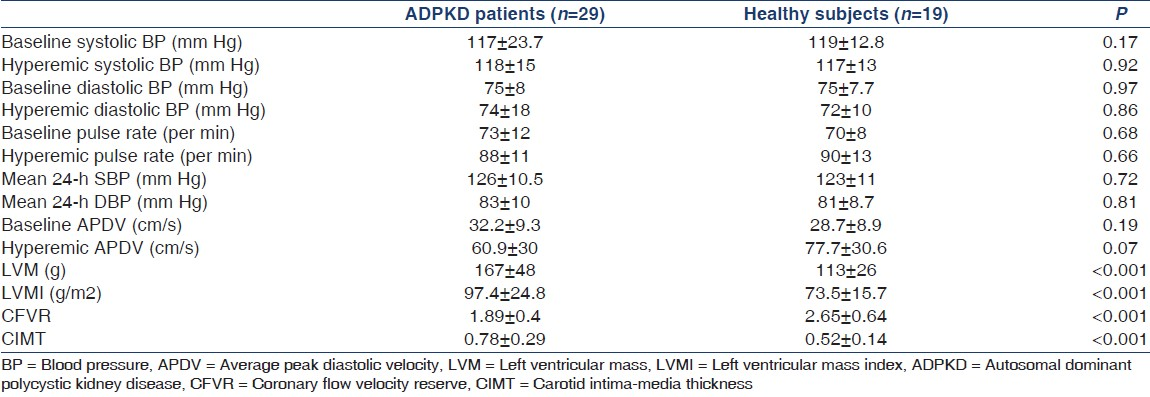
Demographic and anthropometric data of the groups are shown in Table 1. Age, sex, body mass index, and serum levels of glucose, creatinine, LDL cholesterol, HDL cholesterol, triglycerides, CRP, microalbuminuria, and creatinine clearance were similar between ADPKD patients and healthy subjects.
In patients with ADPKD, total cholesterol, neutrophil counts, NLR, CIMT, LVMI, and HOMA-IR were significantly higher and lymphocyte count was significantly lower compared to that in healthy subjects [Tables 1 and 2].
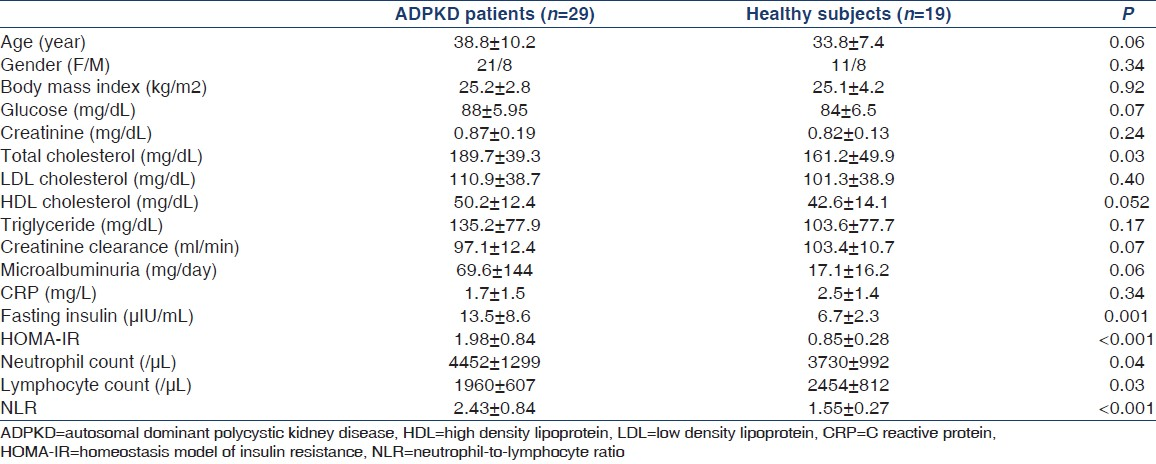
Twenty-four hour ambulatory blood pressure results are shown in Table 2. There was no statistically significant difference regarding both mean systolic and diastolic blood pressure results between patients and healthy subjects.
Regarding the CFVR data, there was no significant difference in baseline and hyperemic APDV values between the groups [Table 2]. CFVR values were significantly lower in patients with ADPKD than in controls [Table 2]. Both CIMT and CFVR results were independent of blood pressure values.
Correlations
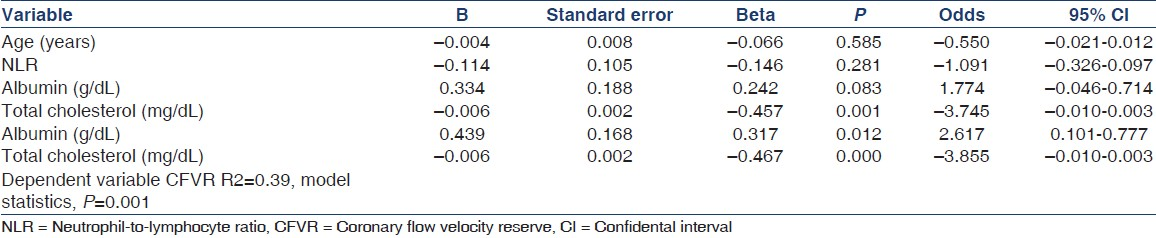
NLR showed positive correlation with CIMT, HOMA-IR, insulin, glucose, total cholesterol, and HDL cholesterol levels, whereas it was inversely correlated with CFVR [Figure 1] and albumin level in all subjects [Table 3]. In the subgroup analyses, NLR showed weak and insignificant positive correlation with total and HDL cholesterol levels and weak and inverse correlation with the LVMI and albumin level in patients with ADPKD [Table 3]. NLR showed moderate and nearly significant positive correlation with albumin in the healthy subjects [Table 3].
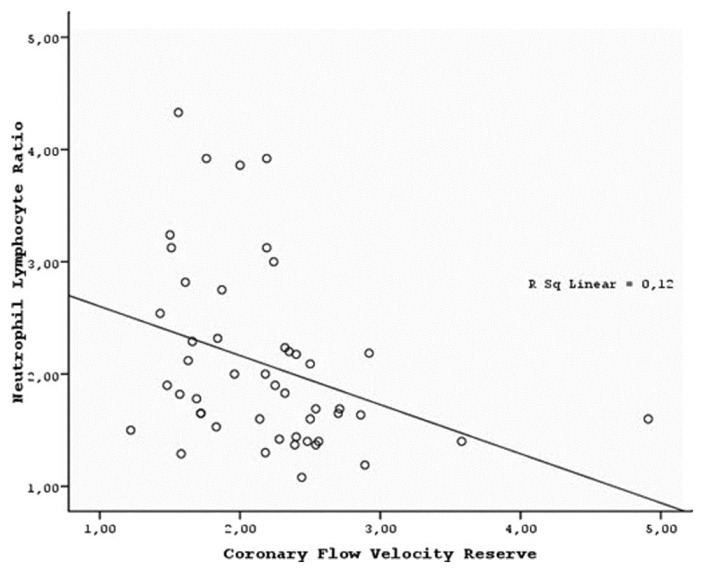
- Linear correlation between NLR and CFVR in all subjects
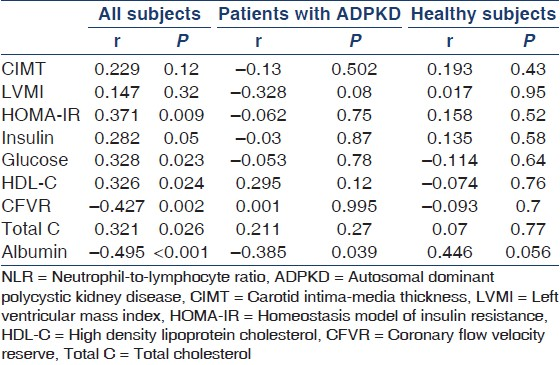
Multivariate linear regression analysis revealed that serum albumin and total cholesterol levels were independent predictors of decreased CFVR, whereas NLR was not [Table 4].
Discussion
There were five main findings in this study. First, absolute neutrophil count and NLR were significantly higher and absolute lymphocyte count was significantly lower in ADPKD patients compared to that in the healthy subjects. Second, NLR was negatively correlated with CFVR and positively correlated with CIMT in all subjects. Third, HOMA-IR values were higher in the ADPKD patients compared to that in the healthy controls, and NLR was associated with HOMA-IR in the whole group. Fourth, total cholesterol and HDL cholesterol levels were positively correlated, and serum albumin levels and LVMI measurements were negatively correlated with NLR in ADPKD patients. Fifth, in multivariate regression analysis, serum albumin and total cholesterol levels were independent predictors of decreased CFVR, whereas NLR was not.
Despite the improvements in the diagnosis and medical treatment of ADPKD, CVD remains the most common cause of death in this population.[1] Pathological findings of subclinical organ damage including LVH, ED, hypertension, microalbuminuria, IR, and increased CIMT may be seen early in the course of ADPKD.[242526] CFVR represents the capacity of the coronary circulation to dilate after an increase in myocardial demands and can be expressed by the difference between the hyperemic flow and the resting flow curve.[27] Decreased CFVR reflects coronary ED and is associated with a significantly higher incidence of cardiovascular events during long-term follow-up period of patients with coronary artery disease.[2829] Reduced CFVR has also been demonstrated in patients with diabetes and without overt coronary artery disease, and in patients with syndrome X.[3031] We have previously showed a significant relationship between increased CIMT, LVM, LVMI, and IR[4] and have also reported the presence of inverse correlation between CIMT and CFVR even in normotensive ADPKD patients with preserved renal functions.[2] These findings are also consistent with previous studies.[63233]
Inflammation was demonstrated as another important finding in ADPKD patients with or without preserved renal function.[10] Previous experimental studies have suggested a role for cytokines in the development of interstitial inflammation leading to kidney injury and progression in ADPKD.[34–36] White blood cell (WBC) count and its subtypes are also known as classic markers of inflammation in CVD.[37] In recent years, the presence of neutrophilia and relative lymphopenia was shown to be an independent predictor of mortality in patients with acute heart failure.[1314] Moreover, NLR was introduced as a novel marker to determine inflammation in cardiac and noncardiac disorders.[15–17] Additionally, NLR was shown as a predictor of long-term mortality in patients who underwent percutaneous coronary intervention.[38]
This study indicated that absolute neutrophil count and NLR were significantly higher and absolute lymphocyte count was significantly lower in patients with ADPKD compared to healthy subjects. These data are in accordance with previous reports suggesting increased inflammatory markers in ADPKD.[1011] However, the CRP level was not correlated with NLR in this study.
NLR was inversely correlated with CFVR in the whole group, suggesting that inflammation may be associated with ED, but this correlation did not exist in either group when the groups were separated. However, whereas CFVR was impaired in 67.9% of the patients with ADPKD, it was normal in all of the healthy subjects. This may have influenced the correlation analyses in either group. Furthermore, the fact that the sample size was small when the groups were separated may explain the absence of significance in correlation analyses.
NLR was also positively correlated with CIMT in the whole group, whereas it was positively correlated with LVMI in patients with ADPKD but not in the whole group. These data also suggest that NLR could be a marker of ED. Further studies with greater sample sizes would be relevant to reveal the possible association between NLR and ED in ADPKD.
In this study, both NLR and HOMA-IR values were higher in the ADPKD group compared to healthy controls, and NLR was associated with HOMA-IR in the whole group. When examined separately, this correlation was absent in either group. As there is a strong link between inflammation, IR, and ED in various disorders, further data are needed for a better understanding of the association of NLR with each of these parameters in patients with ADPKD.
The calculation of NLR is a very simple and inexpensive method compared to the commonly used inflammatory cytokines including IL-6, IL-1β, and tumor necrosis factor (TNF)-α. Therefore, this simple method can be used by internists, nephrologists, and other health care staff as a screening tool for inflammation in ADPKD patients before applying other expensive and invasive procedures.
In a meta-analysis of three studies with long-term follow-up, Stampler et al.[39] provided a summary of epidemiologic evidence supporting the importance of cholesterol control at an early age, and the relative risk of mortality secondary to CAD was particularly for those with the highest levels of total cholesterol. This supports that hypercholesterolemia increases the risk of developing CAD. In this study, serum total cholesterol levels were found to be one of the predictors of decreased CFVR that reflects ED and CAD in ADPKD patients. Limitations of our study include the cross-sectional design and the small sample size. Therefore, these findings cannot be extrapolated to the larger populations.
In conclusion, the global picture of inflammatory cytokines in ADPKD patients is rather complex. NLR that was found to be increased in these patients with ADPKD may be a potential marker of inflammation and ED. Further studies with larger sample sizes evaluating the relationship between NLR and CVD in ADPKD patients are needed.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5:221-8.
- [Google Scholar]
- Coronary flow velocity reserve and carotid intima media thickness in patients with autosomal dominant polycystic kidney disease: From impaired tubules to impaired carotid and coronary arteries. Clin J Am Soc Nephrol. 2008;3:986-91.
- [Google Scholar]
- Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:2244-9.
- [Google Scholar]
- Insulin resistance and coronary flow velocity reserve in patients with autosomal-dominant polycystic kidney disease. Intern Med J. 2012;42:146-53.
- [Google Scholar]
- Hypertension in autosomal-dominant polycystic kidney disease: Early occurrence and unique aspects. J Am Soc Nephrol. 2001;12:194-200.
- [Google Scholar]
- Insulin resistance is related to left ventricular hypertrophy in patients with polycystic kidney disease type 1. Am J Kidney Dis. 2003;41:1219-24.
- [Google Scholar]
- Improvement of coronary flow velocity reserve with telmisartan in patients with autosomal-dominant polycystic kidney disease. South Med J. 2010;103:409-13.
- [Google Scholar]
- Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. Am J Med Sci. 2012;343:46-51.
- [Google Scholar]
- Angiotensin receptor blocker improves coronary flow velocity reserve in hypertensive patients: Comparison with calcium channel blocker. Hypertens Res. 2007;30:699-706.
- [Google Scholar]
- Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:7-13.
- [Google Scholar]
- Peripheral augmentation index and vascular inflammation in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2011;26:2515-21.
- [Google Scholar]
- Inflammatory cytokine profile in autosomal dominant polycystic kidney disease. Contrib Nephrol. 1997;122:35-7.
- [Google Scholar]
- Neutrophilia predicts death and heart failure after myocardial infarction: A community-based study. Circ Cardiovasc Qual Outcomes. 2009;2:656-62.
- [Google Scholar]
- The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24:451-4.
- [Google Scholar]
- Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653-7.
- [Google Scholar]
- Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747-52.
- [Google Scholar]
- Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-4.
- [Google Scholar]
- Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824-7.
- [Google Scholar]
- Homeostasis model assessment: Insulin resistance and betacell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-9.
- [Google Scholar]
- Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation. 1978;58:1072-83.
- [Google Scholar]
- Report of the American Society of Echocardiography Committee on Nomenclature and Standards in Two-dimensional Echocardiography. Circulation. 1980;62:212-7.
- [Google Scholar]
- Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613-8.
- [Google Scholar]
- Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation. 1998;97:1557-62.
- [Google Scholar]
- Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1381-8.
- [Google Scholar]
- Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2004;43:854-60.
- [Google Scholar]
- Coronary flow reserve in stress-echo lab. From pathophysiologic toy to diagnostic tool. Cardiovasc Ultrasound. 2005;3:8.
- [Google Scholar]
- Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-906.
- [Google Scholar]
- Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948-54.
- [Google Scholar]
- Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol. 2007;99:1378-83.
- [Google Scholar]
- Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472-7.
- [Google Scholar]
- Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32:970-5.
- [Google Scholar]
- Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ. 1994;309:1617-8.
- [Google Scholar]
- Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int. 2001;60:2087-96.
- [Google Scholar]
- Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:2588-95.
- [Google Scholar]
- A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med. 2008;14:863-8.
- [Google Scholar]
- Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638-43.
- [Google Scholar]
- Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993-6.
- [Google Scholar]
- Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311-8.
- [Google Scholar]







