Translate this page into:
Occurrence of Neuroendocrine Tumor of Pancreas 20 Years After Kidney Transplant
Corresponding author: Georgi Abraham, Department of Nephrology, MGM Healthcare, Chennai, India. E-mail: abraham_georgi@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Abraham G, Sundar D, Mathew M, Kumar S, Sarangapani A, Ganesamoni S. Occurrence of Neuroendocrine Tumor of Pancreas 20 Years After Kidney Transplant. Indian J Nephrol. doi: 10.25259/IJN_234_2024
Abstract
Neuroendocrine tumor (NET) of the pancreas has not been previously reported in a kidney transplant recipient. We present a 62-year-old lady with a NET of the pancreas 20 years after the transplantation with diarrhea and weight loss. The tumor was enucleated along with removal of liver metastasis, and she remains symptom-free with no recurrence 6 months later.
Keywords
Immunosuppression
Neuroendocrine tumor
Pancreas
Renal transplant
Introduction
Long-term immunosuppressive therapy in kidney transplant recipients leads to immune-related and nonimmune-related complications.1 These complications present as neoplasms, infections, metobolic diseases, and adverse effects of drugs.1,2 The geographical distribution of neoplasms are diverse depending upon gender, genetic predisposition, family history, underlying kidney disease, and dose of immunosupressive drugs.2 Neuroendocrine tumors (NETs) are rare adverse manifestations of transplantation.2 NETs primarily involving pancreas are extremely rare as the presentation is diverse in terms of symptoms, and management requires a multidisciplinary approach.3
Case Report
A 62-year-old lady underwent a live-related kidney transplantation in October 2001 for end-stage-renal disease of unknown etiology. She was on triple immunosupressive therapy including prednisolone, azathioprine, and cyclosporine A. She developed NODAT (new-onset diabetes mellitus after transplantation) in December 2016, and was treated with linagliptin and metformin with good glucose control. She weighed 58.5 kg in November 2022 with the serum creatinine 1.30 mg/dl and no proteinuria. She had episodes of diarrhea in 2018 which responded to symptomatic therapy.
In May 2023, she had many episodes of diarrhea with left periumbilical abdominal pain and fever. Stool examination was unremarkable. Pancreatin was added to the therapy leading to partial relief. She started losing body weight and by October 2023, she weighed 52.4 kg. The diarrhea was intermittent and stool examination was negative for pathogens. The ultrasound of the abdomen was noncontributory. She had undergone upper GI endoscopy and colonoscopy with biopsy which did not reveal the cause of diarrhea.
The contrast CT abdomen showed a lesion in the head of pancreas. Subsequently, 68 Ga DOTA PET CT scan showed focal increased uptake of Ga 68 DOTATATE in the nodular soft tissue lesion probably arising from the head of pancreas. Superiorly, the lesion was abutting the caudate lobe of the liver. No evidence of disease was observed anywhere else in the body [Figure 1a]. PET CT was suggestive of primary NET arising from the head of pancreas with metastatic periportal node. Serum chromogranin was positive.
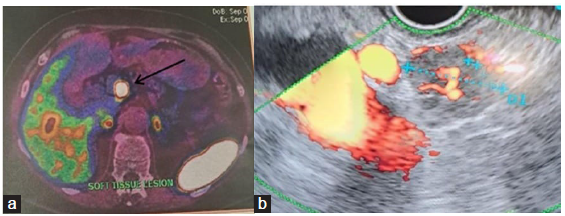
- (a) DOTANOC scan showing pancreas tumor (black arrow). (b) Endoscopic ultrasound doppler and biopsy.
Endoscopic ultrasound-guided biopsy of the pancreatic tumor [Figure 1b]. Immunohistochemical studies revealed cytokeratin +ve, synaptophysin +ve, chromogranin +ve, Ki-67 (antigen kiel 67) 1–2%, TTF-1 (thyroid transcription factor-1) –ve, and CDX-2 (caudal-type homeobox 2) +ve. Oncosurgical opinion had offered two choices: Removal of tumor and Whipple’s procedure or enucleation of the tumor in the pancreas and the left lobe of the liver. Enucleation of pancreatic neck lesion, lymph node dissection, intra-operative ultrasound-guided localization of liver metastases followed by caudate lobe liver resection and segment II/III metastasectomy. The surgery was successful and she was discharged home.
Histopathology of the tumor is shown in Figure 2.
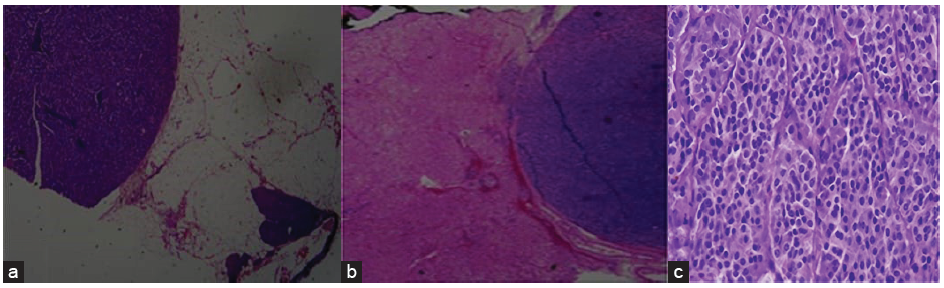
- The histopathology of the tumor (a) showing native pancreatic tissue with NET, (b) liver parenchyma with metastatic pancreatic NET (H&E 2× magnification), (c) well-differentiated pancreatic NET. (H&E 40 x magnification). H&E: Hematoxylin and eosin, NET: neuroendocrine tumor.
Blood sugar control was obtained with insulin analogues. She was continued on pancreatic enzyme supplement and immunosupression with prednisolone 5 mg OD, azathioprine 25 mg OD, and cyclosporine A 50 mg BD (reduced dose). Oncology consultation suggested no further chemotherapy or immunotherapy. The current serum creatinine is 1.4mg/dl. She had no further diarrhea.
During her follow-up 6 months later, she weighed 61 kg and a repeat PET-CT scan showed no focal lesion in the residual pancreas and liver; there was appearance of focal 68 Ga DOTANOC avid nodular lesion in the distal pyloric antrum along the lesser curvature just proximal to the gastroduodenal junction. A gastroenterologist performed an upper GI endoscopy, and the results were negative for any residual tumor.
Discussion
Transplant recipients are at an increased risk of malignancies, the most common being skin cancers and lymphomas. Merkel cell carcinoma, a type of neuroendocrine malignancy of the skin, has been described as a complication after renal transplant.3 Gastrointestinal NET after renal transplant has been reported once previously with rectal involvement and metastasis.3 To our knowledge, we present primary NET of the pancreas with surgical management including enucleation and removal of metastasis as the management option.4
NETs are a rare presentation with immunosupressive therapy. Diagnosis requires a multidisciplinary approach as in our patient. The tumor markers in this patient especially Ki-67 (1–2%) did not suggest an advanced malignancy not requiring chemotherapy. The therapeutic options are based on the type of the tumor, location of the tumor, extent of the tumor, metastasis, and histopathologic classification. The histopathology showed clustered, well differentiated, low-grade tumor in our patient.
This case highlights the importance of pursuing investigations in long-term transplant recipients with diarrhea and weight loss to make the exact diagnosis for targeting therapy.4
Conclusion
We report a rare NET of pancreas with metastasis leading to diarrhea and weight loss in a long-term renal transplant recipient who was on triple immunosuppression. Detailed workup identified the root cause of diarrhea and a multidisciplinary approach was successful.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Long-term complications in renal transplantation. J Am Soc Nephrol. 2000;11:582-8.
- [CrossRef] [PubMed] [Google Scholar]
- Malignancy after renal transplantation. Am J Kidney Dis. 2002;39:E5.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic neuroendocrine tumour in a renal transplant recipient: Dual-tracer PET-CT with 18 F-FDG and 68 Ga- DOTANOC in this rare setting. Nucl Med Mol Imaging. 2015;49:57-60.
- [CrossRef] [PubMed] [Google Scholar]
- Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am J Roentgenol. 2011;197:1221-8.
- [CrossRef] [PubMed] [Google Scholar]







