Translate this page into:
Outcomes of spousal versus related donor kidney transplants: A comparative study
Address for correspondence: Dr. Vinay Sakhuja, Department of Nephrology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012, India. E-mail: vsakhuja2009@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study was designed to compare the outcomes of spousal donor (SD) with related donor (RD) kidney transplants performed at our center between January 2010 and October 2012. A total of 323 adult, ABO-compatible kidney transplants (SD 150 [46.4%], RD 173 [53.6%]) were included. Data on outcomes at 6 months post-transplant was collected retrospectively (2010-2011) and prospectively (January-October 2012). Majority of the donors (SD 88%, RD 72.2%) were females. In the SD group, donors were younger (SD 35.6 ± 8.2 years, RD 45.2 ± 11.5 years; P < 0.0001), whereas recipients were older (SD 42.2 ± 8.3 years, RD 30.0 ± 9.5 years; P < 0.0001). A significantly higher proportion of patients in the SD group were given induction therapy (43% vs 12%; P < 0.001). Biopsy proven acute rejections were more common in the RD group (16% vs 28.3%; P = 0.01). Majority (80.8%) of the acute rejections occurred in the first 2 weeks post-transplant in both groups. Isolated acute cellular rejections (ACRs) and isolated antibody mediated rejections constituted 50% and 25% of rejection episodes in both groups, whereas the remainder had histological evidence of both. The proportion of steroid responsive ACRs was similar in both groups (SD 83.3%, RD 65.4%; P = 0.2). The number of patients with abnormal graft function at the end of the study was higher in the RD group (2.3% vs. 12.3%; P = 0.001). Patient survival and infection rates were similar in the two groups. We conclude that short-term outcomes of SD transplants are not inferior to RD transplants. Lesser use of induction therapy in the RD group may explain the poorer outcomes as compared to the SD group.
Keywords
Kidney transplant
outcomes
related donor
spousal donor
Introduction
Kidney transplantation is the treatment of choice for end stage renal disease as it provides the best hope for rehabilitation to normal life in these patients. Although the number of deceased donor transplants is increasing, living donor transplants constitute the vast majority of all kidney transplants in India.
In a living donor transplant program, spousal donors (SDs) are necessary to fulfill the huge gap between the demand and availability of donors. However, outcomes of transplants from these “biologically unrelated” donors need to be rigorously evaluated before they can be considered to be at par with living related donor (RD) transplants. Although a number of previous studies[1234567891011121314] have shown that outcomes of SD transplants are similar to living RD transplants, only few studies from India[151617] have looked into this aspect. In addition, the majority of previous studies were done using cyclosporine and azathioprine, drugs which are infrequently used as first line agents currently. This study was, therefore, designed to assess the graft and patient outcomes of SD transplants and to compare them with those of RD transplants.
Patients and Methods
Adults aged 18 years or above who underwent renal transplantation from a SD or RD between January 2010 and October 2012 were included in the study. Patients undergoing deceased or living unrelated donor (other than SD) transplantation were excluded from the study. Patients undergoing a second renal transplant, those with prolonged cold ischemia times (greater than 2 h), those non-compliant with medicines and those lost to follow-up were also excluded. Data on outcomes at 6 months post-transplant was collected retrospectively (2010-2011) and prospectively (January-October 2012).
Pre-transplant evaluation and post-transplant management protocol
Pre-transplant cross-match was done using complement dependent cytotoxicity (CDC) technique only. Induction therapy with basiliximab/antithymocyte globulin (ATG) was administered to patients (if affordable) if considered at high-risk of acute rejection (based on the physician's discretion). Basiliximab was given at 20 mg intravenous (i.v.) 2 h before transplantation and 20 mg i.v. at day 4 post transplant. ATG was given at 1 mg/kg i.v. on day 0, 1 and 2 post-transplant. Maintenance immunosuppression was a combination of calcineurin inhibitor (tacrolimus was used in >90% of patients), mycophenolate mofetil (MMF) and steroid. All patients received 500 mg of hydrocortisone at the time of clamp release intra-operatively and 20 mg of prednisolone orally from post-operative day 2. Prednisolone was tapered to 5 mg once daily over the next 2 months. Tacrolimus trough levels were targeted at 12-15 ng/ml in the 1st week post-transplant, 10-12 ng/ml at week 2-4 post-transplant, 8-10 ng/ml at 1-3 months post-transplant and 5-8 ng/ml thereafter. All patients received MMF 1 g twice a day. The calcineurin inhibitor and MMF were started 24 h before transplant. Trimethoprim-sulfamethoxazole prophylaxis was given to all patients for 6 months after transplant.
Testing for cytomegalovirus (CMV)/BK virus was done using nucleic acid testing/allograft biopsy when clinically indicated. Prophylactic or preemptive therapy for CMV was not given. Testing for CMV serostatus prior to transplant was not done.
Delayed graft function was defined as the requirement of dialysis in the 1st week after transplant. Evaluation for an acute rise in serum creatinine level included ultrasonography, calcineurin inhibitor C0 level and urine microscopy and culture. If no obvious cause of renal dysfunction was identified, a renal biopsy was performed. Rejection was classified as per Banff classification.
Acute cellular rejection (ACR) was treated with 3 pulses of methyl prednisolone (500 mg each). In case of steroid resistance, ATG was administered (1 mg/kg/d for 5-7 pulses). Antibody mediated rejection (AMR) was treated with plasmapheresis (PP) (40-60 ml/kg/session; replacement with fresh frozen plasma/albumin) and IvIg (100 mg/kg after each PP session). Combined ACR and AMR were treated with an initial steroid pulses followed by ATG (if steroid resistant). PP/IvIg therapy was also used in some cases depending on the treating physician's discretion. Response to therapy was classified as a complete response (CR) if serum creatinine decreased to ≤1.5 mg/dl or baseline after therapy, partial response if serum creatinine decreased by 50% of maximum, but was still >1.5 mg/dl or baseline and no response if there was a less than 50% decrease in serum creatinine and serum creatinine >1.5 mg/dl on follow-up.
Results
A total of 509 renal transplants were performed at our center during the study period. Amongst those for whom complete follow-up was available (n = 368, 72.3%), SDs constituted 40.8% (n = 150), making them the single largest donor group. Parents, siblings, children and grandparents constituted 31.3% (n = 115), 12.2% (n = 45), 3.0% (n = 11) and 0.5% (n = 2) of all donors respectively.
After excluding cadaver and unrelated donor transplants (other than spouses), 323 patients (SD, n = 150 [46.4%]; RD, n = 173 [53.6%]) were included for the final analysis. Of the SDs, 88% (n = 132) were wives and 12% (n = 18) were husbands. Amongst the recipients, the proportion of females (SD, n = 18 [12%]; RD, n = 29 [16.8%]; P = 0.27) was not significantly different in the two study groups. The recipients in the SD group were significantly older than the RD group (mean age SD 42.2 ± 8.3 years, RD 30.0 ± 9.5 years; P < 0.0001). The donors in the SD group were significantly younger (mean age SD 35.6 ± 8.2 years, RD 45.2 ± 11.5 years; P < 0.0001) and had a higher proportion of females (SD, n = 132 (88%); RD, n = 125 [72.2%]; P = 0.001) than the RD group.
A higher proportion of patients in the SD group received induction when compared with the RD group (SD, n = 65, 43.3%; RD, n = 20, 11.6%; P < 0.001). Of the patients who received induction, 31 (47.7%) and 11 (55%) patients received ATG in the SD and RD groups respectively; the rest received basiliximab. There was no difference in the proportion of patients who were initiated on tacrolimus based immunosuppression (as compared to cyclosporine based immunosuppression) (SD 92.3%, RD 91.3%; P = 0.69) or in the proportion of patients in whom immunosuppression was changed during the follow-up (SD 12.7%, RD 9.2%; P = 0.37) in the two study groups.
Delayed graft function occurred in 15 (4.6%) of the 323 patients and was not significantly different between the SD and RD groups (P = 0.43). Allograft biopsies showed an evidence of rejection in 11 (73.3%) of these patients.
A total of 73 (22.6%) of the 323 patients had an acute rejection episodes. Approximately, half of the rejections in either group were isolated ACRs [Table 1]. Isolated AMR and combined ACR and AMR each accounted for about one-fourth of the rejections. Majority (n = 59; 80.8%) of the rejection episodes occurred within the first 2 weeks after transplant in both study groups. Acute rejection rates were higher in the RD group as compared to the SD group (SD 16%, RD 28.3%; P = 0.01). Subgroup analysis of proportion of patients with acute rejection episodes in the two study groups who had received induction therapy (SD 12.3%, RD 30%; P = 0.06) as well as those who did not receive induction therapy (SD 18.8%, RD 28.1%; P = 0.11) revealed that the difference in rejection rates was no longer significant [Table 1]. Within the SD transplant recipients, rejection rates were lower in patients who received induction therapy as compared to those who did not; the difference however was not statistically significant (induction-12.3%, no-induction-18.8%; P = 0.28). In the RD transplant recipients, the rejection rates were similar in the induction and no induction groups (induction-30%, no-induction-28%; P = 0.86) [Table 1].

Majority (27 out of 39 [69.2%]) of the isolated ACRs were steroid responsive (i.e. achieved CR after therapy) [Table 2]. In one patient, serum creatinine returned to normal without any form of therapy. 11 (28.2%) ACRs were steroid resistant (i.e., did not achieve CR after therapy), of which 4 were treated with ATG. Serum creatinine returned to normal in all 4 of these patients. Seven (17.9%) patients with steroid resistant ACR could not afford ATG. The proportion of steroid responsive ACRs was similar in both study groups (SD 83.3%, RD 65.4%; P = 0.2).

Of the 12 combined ACR and AMRs, 8 (66.7%) responded to steroids alone, whereas, in 3 (25%) cases creatinine returned to normal only after ATG therapy. One patient with combined ACR and AMR had steroid and ATG resistant rejection. In isolated AMRs 11 of the 20 (55%) patients did not receive any additional immunosuppressive therapy. Therapy and response of the remaining 9 patients is shown in Table 2. Two patients had borderline rejection, both of which responded to steroids.
Death censored deranged graft function, defined as serum creatinine >1.5 mg/dl at the end of follow-up, occurred in significantly more number of patients in the RD group in comparison to the SD group (12.3% vs. 2.8%; P = 0.001). Proportion of patients with deranged graft function was lower with the use of induction therapy (induction-4.8%, no-induction-9.1%; P = 0.2); although, the differences were not statistically significant.
A total of 7 (2.2%) patients expired during the study period (RD, n = 2; SD, n = 5; P = 0.26). The causes of death were bacterial sepsis (four patients), mucormycosis (one patient), decompensated hepatitis C related chronic liver disease (one patient) and hyperacute rejection followed by disseminated intravascular coagulation (one patient).
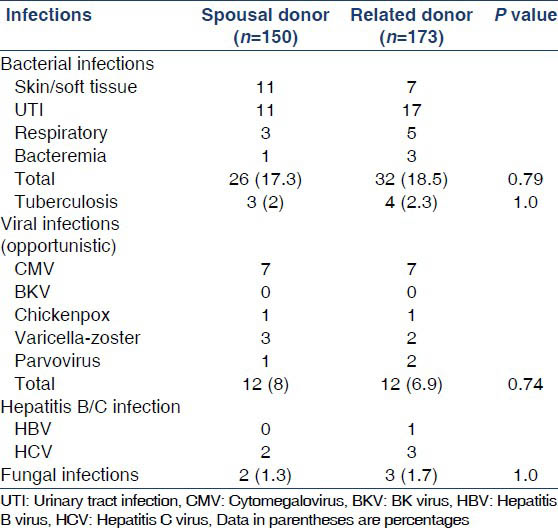
Bacterial infections were the most common infections with about 18% of recipients having a bacterial infection during the study period. Urinary tract infections were the commonest bacterial infections in both groups, followed by skin/soft-tissue infections and respiratory tract infections [Table 3]. 2.2% of patients had post-transplant tuberculosis. CMV was the commonest viral infection occurring in about 4.4% of the patients. There was no difference in the occurrence of bacterial, tubercular, viral or fungal infections between the two study groups.
Discussion
Kidney transplantation remains a distant dream for most of the Indian patients, with only about 2% of all patients being worked-up for transplantation[18] and deceased donor transplants account for less than 5% of all kidney transplants.
Presently, kidney transplantation in India is regulated by the Transplantation of Human Organs and Tissues Act, 1994 and its amendments. The Act confers the status of a “near relative” to a SD, thereby making it much easier for spouses to donate organs than other biologically distant relatives as well as unrelated donors. This significantly enlarges the pool of available donors and makes SDs the single largest donor group at many centers. This fact is also borne out in the present study with SDs constituting about 41% of all donors.
In the present study, the mean age of donors in the RD group was significantly higher than that in SD group (45.2 vs. 35.6 years), reflecting the fact that the majority of the donors in the RD group were parents. Majority (73.7%) of the recipients in this study did not receive induction therapy, particularly in the RD group where only 11.6% received induction. This is a cost reduction strategy and in turn reflects the relatively poor economic status of our patient population.
In the present study, recipients of grafts from RDs were found to have a higher rate of rejection (and poorer graft function at the end of follow-up) as compared to those who received kidneys from SDs. This is in contrast to previously published studies, where the incidence of an acute allograft rejection has been found to be slightly more in SD transplants as compared to RD transplant[124791013151619] although the difference has been found to be statistically significant in only one study.[10] One study[8] has found that recipients of SD transplants are significantly more likely to have early acute allograft rejection as well as more severe rejection. Similarly, another study[1] has reported graft function in SD transplant recipients to be significantly inferior at the end of the follow-up period when compared to RD transplant group. In other studies[2478911151619] however, graft function was similar in the two groups. Possible reasons for the contrasting results are:
-
Use of induction therapy: Present evidence in literature strongly supports the use of induction therapy as a part of the initial immunosuppressive regimen in kidney transplant recipients.[2021222324] In a Cochrane review of 30 randomized controlled trials (RCTs) on the efficacy and safety of IL-2 receptor antagonists,[20] it was shown that these agents decrease the rate of acute rejection (relative risk [RR] =0.77; 0.64-0.92) and graft loss (RR = 0.74, 0.55-0.99), without significantly affecting all-cause mortality, malignancy or infection rates. Similarly, a meta-analysis of RCTs comparing lymphocyte depleting agents with placebo or no treatment showed a reduction in graft failure (RR = 0.66, 0.45-0.96),[22] particularly in high immunological risk patients. Although, the use of lymphocyte depleting agents results in lower acute rejection rates, they are associated with more infections and malignancy.[23]
Primarily in an effort to reduce transplant costs, induction therapy was used in only a minority of patients in the present study, particularly so in the RD transplant group (RD 11.6%; SD 43.3%). To some extent, this also reflects the treating physician's (apparently false) sense of reassurance that the RD transplants are at relatively lower immunological risk, thereby preventing them from using induction therapy. This might explain the overall high rejection rates observed in the present study and also the fact that acute rejection rates were significantly more frequent in the RD group. Sub-analysis of recipients in both the RD group as well as the SD group who received induction in our study showed that the rate of rejection was not different from those who did not receive induction, although the number of patients in the sub analysis groups was too small to make reliable conclusions. Another single center study from India[25] and a previous study from our center[26] did not find a significant difference in acute rejection rates with or without induction therapy, probably again reflecting a lack of adequate power of these studies to detect a difference as in the present study.
-
Pre-transplant cross-match technique: Currently, cross-match is performed only by the CDC technique at our center. This technique is less sensitive in detecting circulating donor specific antibodies as compared to newer techniques (flow-cytometry/luminex). Patients who are cross-match positive by one of these newer techniques may have higher acute rejection rates although they may be cross-match negative by the CDC.[27] This might be one of the factors contributing to the higher rejection rates in the present study.
Overall patient survival at the end of follow-up was 97.8% in the present study. Patient survival in the two study groups was similar. The results of the present study are similar to the previous studies where patient survival has been found to statistically similar in the spousal and related transplant recipient groups.[1247912]
As apparent, a significant limitation of our study was the fact that the two study groups were heterogeneous in terms of induction therapy and donor age, factors which are known to affect acute rejection rates and graft function on follow-up. Although a subgroup analysis of patients who received induction therapy (as well as those who did not) in the groups did not reveal any significant difference, this could possibly be because of small numbers in the subgroups. In addition, the study has a short duration of follow-up and also has the inherent constraints of retrospectively collected data, with some patients being lost to follow-up.
We conclude that outcomes of SD renal transplants are not inferior to those of RD transplants in our center. Greater use of induction therapy may help in improving outcomes at our renal transplant program.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Living-unrelated donor renal transplantation: An alternative to living-related donor transplantation? Ann R Coll Surg Engl. 2008;90:247-50.
- [Google Scholar]
- Kidney transplantation from related and unrelated living donors in a single German centre. Nephrol Dial Transplant. 2003;18:418-25.
- [Google Scholar]
- Outcome in emotionally related living kidney donor transplantation. Nephrol Dial Transplant. 1997;12:1940-8.
- [Google Scholar]
- Spousal renal donor transplantation in Chinese subjects: A 10 year experience from a single centre. Nephrol Dial Transplant. 2004;19:203-6.
- [Google Scholar]
- Outcomes of renal transplants from spousal donors: 25 years of experience at our center. Int J Artif Organs. 2010;33:40-4.
- [Google Scholar]
- Living kidney transplantation between spouses: Results in 100 cases. Transpl Int. 1994;7(Suppl 1):S314-7.
- [Google Scholar]
- Living unrelated donors in kidney transplants: Better long-term results than with non-HLA-identical living related donors? Transplantation. 2000;69:1942-5.
- [Google Scholar]
- Increased rejection in living unrelated versus living related kidney transplants does not affect short-term function and survival. Transplantation. 2004;78:1030-5.
- [Google Scholar]
- Living-unrelated renal transplantation provides comparable results to living-related renal transplantation: A 12-year single-center experience. Surgery. 1996;119:538-43.
- [Google Scholar]
- Unrelated living donors in 141 kidney transplantations: A one-center study. Transplantation. 1998;66:49-52.
- [Google Scholar]
- Kidney transplantation from living donors genetically related or unrelated to the recipients: A single-center analysis. Transplant Proc. 2012;44:1892-6.
- [Google Scholar]
- Kidney transplantation of living unrelated donor-recipient combinations. Transplant Proc. 2012;44:254-6.
- [Google Scholar]
- Comparison of spousal with other donor groups: Study of a single center. Transplant Proc. 2006;38:562-3.
- [Google Scholar]
- Long-term outcomes of renal transplants from spousal and living-related and other living-unrelated donors: A single center experience. J Assoc Physicians India. 2012;60:24-7.
- [Google Scholar]
- The spouse as a donor in renal transplants. Saudi J Kidney Dis Transpl. 2006;17:77-81.
- [Google Scholar]
- Spousal renal transplants: Implications in developing countries. Transplant Proc. 2003;35:26-7.
- [Google Scholar]
- What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Comparison of long-term outcomes between spousal transplants and other living unrelated donor transplants: Single-center experience. Nephron Clin Pract. 2009;113:c241-9.
- [Google Scholar]
- Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation. 2004;77:166-76.
- [Google Scholar]
- The cost-effectiveness of induction immunosuppression in kidney transplantation. Nephrol Dial Transplant. 2009;24:2258-69.
- [Google Scholar]
- Effect of anti-lymphocyte induction therapy on renal allograft survival: A meta-analysis. J Am Soc Nephrol. 1997;8:1771-7.
- [Google Scholar]
- Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967-77.
- [Google Scholar]
- Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
- [Google Scholar]
- Comparison of azathioprine with mycophenolate mofetil in a living donor kidney transplant programme. Indian J Nephrol. 2011;21:258-63.
- [Google Scholar]
- Efficacy of Basiliximab induction in poorly matched living donor renal transplantation. Ind J Nephrology. 2013;23:409-12.
- [Google Scholar]
- Understanding crossmatch testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology (Carlton). 2011;16:125-33.
- [Google Scholar]







