Translate this page into:
Paired kidney exchange transplantation: Maximizing the donor pool
Address for correspondence: Dr. Pranaw Kumar Jha, Department of Nephrology, Medanta Kidney and Urology Institute, Medanta-The Medicity, Gurgaon - 122 018, Haryana, India. E-mail: dr.pranaw@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the last decade, paired kidney exchange (PKE) transplantation has gained popularity worldwide as a viable alternative for end stage renal disease (ESRD) patients who have incompatible or sensitized donors. This study presents our experience with PKE transplantation and compares outcome between PKE and non-PKE renal transplant recipients. Between February 2010 and November 2013, 742 transplants were performed, of which 26 (3.5%) were PKE transplantations. All were two-way exchanges. PKE recipients were significantly older than non-PKE (46.73 ± 9.71 vs. 40.08 ± 13.36 years; P = 0.012) while donor ages were comparable. PKE patients had significantly higher number of HLA mismatches (5.03 ± 1.14 vs. 3.49 ± 1.57; P < 0.0001). After a median follow-up of 20 months (range: 3–47 months), there was no significant difference in patient survival (PKE 96.16% vs. non-PKE 96.65%; P = 0.596) and death censored graft survival (PKE 96.16% vs. non-PKE 96.37%; P = 1). Mean serum creatinine at 1 month and at last follow-up was lower in PKE versus non-PKE group (0.98 ± 0.33 vs. 1.3 ± 0.61 mg/dl; P = 0.008 and 0.96 ± 0.30 vs. 1.27 ± 0.57 mg/dl, P = 0.006, respectively). Biopsy proven acute rejection rate was 11.5% in PKE group and 16.89% in non-PKE patients (P = 0.6). To conclude, paired kidney donation is an excellent way of increasing the donor pool and needs to be promoted to overcome the shortage of suitable kidney in our country.
Keywords
Developing world
India
kidney transplant
paired kidney exchange
Introduction
Kidney transplantation is the best form of renal replacement therapy (RRT) for end stage renal disease (ESRD) patients.[1] In India, of 175,000 new chronic kidney disease (CKD) patients developing ESRD annually, <10% receive any form of RRT and only 2% undergo renal transplant.[2] As per the first report of Indian CKD registry, of all the stage 5 CKD cases, 39% were on RRT out of which only 2% were being worked up for renal transplant.[3] Although deceased donation is popular in western countries, it is still in its nascent stage here. Due to the uncertainty and long waiting time compounded with lack of awareness among general public, deceased donor transplantation is not a very popular treatment option yet for ESRD patients. Also, there are regional variations in deceased donor transplantation with majority happening in southern states of India and very few in northern states. Although outcomes of living donor transplantations are better than deceased donor transplants, there are very few living voluntary related donors who come forward for the noble cause. Many potential living donors get rejected due to ABO incompatibility or a positive cross-match with intended recipient. Options left in such a scenario are ABOi transplants and desensitization of sensitized recipient or paired kidney exchange (PKE) transplantation. Former is considerably more expensive and requires more immunosuppression while PKE is economically much better option, requires less immunosuppression compared to ABOi transplants and desensitization protocols, and is legally valid as well.
Indian experience in this field has been far and few.[4] We hereby present experience of PKE transplantation at our institution, a tertiary care center in north India.
Materials and Methods
This is a retrospective analysis of consecutive renal transplants performed at our center between February 2010 and November 2013. Total 742 renal transplants were performed during the study period. All the patients who underwent renal transplant during this time period were included. Patients were divided into two groups-PKE group and other patients (i.e., non-PKE group). Medical records of these patients were reviewed extensively.
A paired kidney registry is being maintained at our center and various incompatible pairs were matched depending upon the availability of suitable donors and compatible recipients. Matching and donor allocation was done manually. As far as possible, donors were matched for age and glomerular filtration rate. Donor investigations were completed once the matching was done. When the pair was from state other than Haryana, necessary clearance was obtained from authorization committee of that state as well. Both the pairs were counseled in detail. It was also emphasized that occasionally one of the kidneys might not function as well as the other one in such PKE transplantation. Necessity of performing donor nephrectomies simultaneously in two different operation theaters to avoid reneging was explained. Appropriate investigations, including various radiological, biochemical and serological tests were done as per standard protocol including diethylene triamine penta-acetic acid renogram and computed tomography renal angiogram of the donor. Necessary clearances were obtained. Donors and recipients were allowed to meet before renal transplant. Complement-dependent cytotoxicity (CDC) cross-match was done for all the patients while flow cytometry was done when indicated. All donors were operated by laparoscopic nephrectomy. Donor nephrectomies were performed simultaneously in different theatres.
Induction was offered to all the patients. Immunologically high-risk recipients (i.e., history of multiple blood transfusions pre-transplant, second or more renal transplant, multiple pregnancies, wife recipient) were offered thymoglobulin (1 mg/kg/day IV for 3 days) while others were offered basiliximab (20 mg intravenous on the day of transplant and repeated on postoperative day 4). Injection methyl prednisolone 500 mg IV was given intraoperatively to all the patients followed by 40 mg/day oral prednisolone on day 1, which was tapered to 20 mg by day 8. Maintenance immunosuppression consisted of a calcineurin inhibitor (tacrolimus or cyclosporine), mycophenolate sodium and prednisolone.
All the patients were followed up in OPD twice weekly for 1st month, once weekly for 2nd month, once in a fortnight for 3rd month and thereafter monthly once for 12 months post-transplantation. Follow-up after 1st year was once in 2–3 months. Renal function tests, including serum creatinine and hemogram were done on every visit. Tacrolimus/cyclosporine level was done as per the need, decided by the treating physician. Tacrolimus level target was 8–12 ng/ml duringfirst 3 months, 5–8 ng/ml from 3 to 6 months and <5 ng/ml thereafter. In patients on cyclosporine, C0 target level was 250–350 ng/ml duringfirst 3 months, 100–250 ng/ml from 3 to 6 months and <100 ng/ml thereafter while C2 target level was 1000–1200 ng/ml duringfirst 3 months and 600–1000 ng/ml thereafter. Both C0 and C2 levels were done for all the patients receiving cyclosporine. Prednisolone was tapered to 10 mg by the end of 3 months and 5 mg by the end of 6 months. Mycophenolate sodium was initiated at 720 mg twice daily initially and tapered to 360 mg twice daily by 6 months. Data were collected retrospectively from medical records, including demographic data, follow-up serum creatinine, biopsy proven acute rejections, graft, and patient loss and infections.
Statistical analysis was done using MedCalc for Windows, version 12.7.8 (MedCalc Software, Belgium). Data were reported as mean values ± standard deviation. Continuous variables were compared using unpaired t-test while categorical values were compared using Chi-square test or Fisher's exact test. Kaplan–Meier method was used to generate survival curves. P < 0.05 was considered statistically significant.
Results
During the study period, total 79 patients got registered for PKE. Of this two were HLA incompatible while the remaining 77 got registered due to blood group incompatibility. No blood group compatible pair got registered for PKE. Of the registered patients, 26 underwent PKE. This constituted 3.5% of total renal transplants (n = 742) performed during the study period. Reason for exchange was ABO incompatibility in all of them. All were two-way donations. Median waiting time for getting suitable donor after registration was 3 months. There were 13 recipients each of blood group A and B. Median follow-up duration was 20 months (range: 3–47 months). Out of the 26 PKE patients, 22 were from other states. Median time from getting a suitable pair to transplant was 2 months, including authorization committee clearance. Of the remaining 53 patients who got registered but could not undergo PKE, 7 patients got transplanted outside, 24 were still waiting for transplant, 18 patients were lost to follow-up, 2 underwent ABO incompatible renal transplant while 2 patients expired.
Table 1 shows the patients' demographic data. Mean recipient age was significantly higher in PKE group (46.73 ± 9.71 years) versus non-PKE group (40.08 ± 13.36 years) (P = 0.012). Donor age was comparable between the two groups (46.53 ± 9.74 years in PKE vs. 48.10 ± 11.22 years in others; P = 0.481). Among PKE recipients, 88.46% (n = 23) were male while 81.14% (n = 582) of patients in non-PKE group were male (P = 0.448). Dialysis vintage was 4.90 ± 2.77 months in PKE versus 4.88 ± 7.49 months in non-PKE group (P = 0.989). Significantly more patients in PKE group had diabetic CKD as cause of ESRD (50% vs. 24.72%, P = 0.009), while there were significantly more patients with chronic glomerulonephritis in non-PKE group (0% vs. 14.39%, P = 0.038). Significantly more patients in PKE group had pre-transplant hepatitis B (11.54%, vs. 2.51%) (P = 0.033). Distribution of hepatitis C was similar between the groups (7.69% in PKE vs. 3.07% in non-PKE; P = 0.203). Twenty-four patients undergoing PKE received basiliximab induction followed by tacrolimus, mycophenolate mofetil and prednisolone as maintenance immunosuppression while the remaining two received thymoglobulin induction followed by tacrolimus and mycophenolate maintenance immunosuppression.
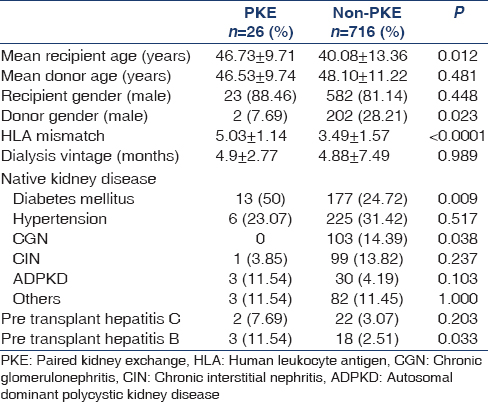
Table 2 shows blood group distribution among the pair registered for PKE. It also shows the blood group distribution and recipient donor relationship of PKE recipients.

Table 3 shows the immunosuppression and surgical details of PKE group patients.

Table 4 shows study outcomes. Mean serum creatinine at one month was significantly better in PKE (0.98 ± 0.33 mg/dl) versus other group (1.3 ± 0.61 mg/dl) (P = 0.008). Same was the case with mean serum creatinine of the two groups at last follow-up (0.96 ± 0.30 mg/dl in PKE vs. 1.27 ± 0.57 mg/dl in non-PKE; P = 0.006). Patient survival was 96.16% in PKE versus 96.65% in non-PKE group (P = 0.596). One patient in PKE group died due to cardiac event 11 months post-transplant. He had past history of coronary artery disease for which coronary artery bypass graft was done before transplantation. Overall graft survival and death censored graft survival rate was 92.31% and 96.16% in PKE while it was 94.27% and 96.37% in other group, respectively (P = 0.658 and 1, respectively). Biopsy proven acute rejection was seen in 11.5% in PKE and 16.89% in non-PKE group patients (P = 0.6). Infection rate was 11.5% in PKE and 8.8% in non-PKE (P = 0.497).

Figures 1 and 2 show Kaplan–Meier curves comparing patient survival and death censored graft survival between the study groups, respectively.

- Kaplan-Meier graph comparing patient survival between PKE group and non-PKE group patients

- Kaplan-Meier graph comparing death censored graft survival between PKE group and non-PKE group patients
Discussion
In the current study, donor age was 46.53 ± 9.74 years, which was comparable to that of other paired kidney donation studies by Tuncer et al. (45 years)[5] and Gumber et al. (45 years).[6] Mean recipient age was 46.73 ± 9.71 years, which was relatively higher compared to above two studies (40.9 years and 36 years, respectively). Mean waiting time on dialysis was 4.9 ± 2.77 months. Patient survival was 96.16% in our study. In the study by Montgomery et al. and Tuncer et al. it was 100%.[75] Similarly, excellent patient survival rate was seen in other studies by Waigankar et al. (100%)[8] and Kute et al. (94.64%).[9] Death-censored graft survival rate was 96.16%, which was similar to excellent graft survival noted in other studies by Montgomery et al. (95.5%),[7] Gumber et al. (94.4%),[6] Pahwa et al. (100%)[10] and Kute et al. (97.5%).[9] Acute rejection rate was 11.5%. This was better than acute rejection rate of 18% reported by Montgomery et al.,[7] 30% by Fuller et al.[11] and 22.8% by Tuncer et al.[5] In studies by Kute et al. and Pahwa et al., acute rejection rate was 16% and 15.9%, respectively.[910]
Despite significantly higher HLA mismatches and older recipients, outcome of PKE recipients in terms of graft function, patient survival and death censored graft survival as well as biopsy proven acute rejection rates were similar to live renal transplant recipients in non-PKE group in our study. Although, the short-term follow-up is an important limiting factor. In a similar study by Kute et al., the outcomes were comparable between PKE and non-PKE group. Here, once again, the follow-up was short term.[12]
There is a huge demand and supply mismatch for living-related donors. Many of the voluntary living related donors get rejected because of ABO incompatibility or positive cross-match. It has been shown that such incompatibilities can account for rejection of 35% of otherwise suitable donors.[13] To circumvent this problem, various alternatives have been devised such as ABOi transplants and desensitization protocols for patients with a positive cross-match. Patients undergoing desensitization or ABOi transplants require plasmaphereis sessions, which expose them to higher chances of blood-borne infections. Also, net immunosuppression is considerably higher in such transplants due to requirement of rituximab, thymoglobulin, etc., over and above regular maintenance immunosuppression. Finally, these options are significantly more expensive compared to conventional transplants. There has been an increasing enthusiasm to promote PKE program as an alternative, which also reflects in the latest amendment of Transplantation of Human Organs (THO) Act 2011. PKE is cheaper and requires less immunosuppression compared to ABOi transplants or desensitization protocols. There is a need of national level PKE program with a national PKE registry to promote better matching and increase number of patients benefiting from it.[14]
Historically, first PKE transplant was performed in South Korea in 1991[15] although it was first proposed by Felix Rappaport way back in 1986.[16] Switzerland and USA performed theirfirst PKE in 1999 and 2000, respectively.[1718] Since then, it has gained popularity across the globe.
Although PKE is an attractive option, there are few ethical issues that need to be addressed. It is difficult to maintain anonymity, which can give rise to coercion and money laundering practices.[19] Financial conflict of interest can arise in case of different socioeconomic status of pairs. Also, there is always a concern among the pairs regarding age difference between the donors and 'quality' of the kidney. Hence, both the parties should be counseled in detail and adequately informed to allay any unnecessary fear and misconceptions. Operations should be performed simultaneously to prevent the problem of reneging by either donor after nephrectomy of the other.[20]
There are some limitations to current study. We did 26 successful PKE transplants, but could not match remaining pairs registered for paired exchange. Matching was done manually. Also, the final number of patients in PKE group for analysis was small. As the number of pairs registered under such program increases, so does the chance of getting a successful match. Hence it is important to promote the program at state and national levels, so that different centers can share the pairs for the benefit of maximum number of recipients. One important hindrance here is that if both the donor nephrectomies are performed at one center, it may not be acceptable to the other center sharing the pair. An alternative is to transfer the kidneys across the centers after nephrectomy. It has been shown that risk of delayed graft function does not increase during such transfer.[21]
Another important limitation is that O blood group recipients are at disadvantage in PKE transplantation because of excess of registered O group recipients compared to very few O group donors. Compatible pairs with O donors should be educated that donation by them is going to help needy O group recipients and increase chances of matching. This has been stressed upon in previous studies as well.[22] A non-directed donation or a deceased organ donation can also help in such a scenario by initiating a domino. Participation by pairs with AB blood group recipients is another way to increase matching.
Although there are few limitations as mentioned above, there is an increasing interest in PKE. There is a growing need to maintain a state and nationwide registry to promote PKE across different centers. This will benefit many patients, expand the donor pool, and avert the need for expensive desensitization protocols and ABO incompatible transplants.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of afirst cadaveric transplant. N Engl J Med. 1999;341:1725-30.
- [Google Scholar]
- Five decades of Indian nephrology: A personal journey. Am J Kidney Dis. 2009;54:753-63.
- [Google Scholar]
- What do we know about chronic kidney disease in India:First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Living donor paired-kidney exchange transplantation: A single institution experience. Indian J Urol. 2010;26:511-4.
- [Google Scholar]
- Comparison of paired exchange kidney transplantations with living related kidney transplantations. Transplant Proc. 2012;44:1626-7.
- [Google Scholar]
- Transplantation with kidney paired donation to increase the donor pool: A single-center experience. Transplant Proc. 2011;43:1412-4.
- [Google Scholar]
- Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA. 2005;294:1655-63.
- [Google Scholar]
- Living donor transplant options in end-stage renal disease patients with ABO incompatibility. Indian J Urol. 2013;29:114-8.
- [Google Scholar]
- Increasing access to renal transplantation in India through our single-center kidney paired donation program: A model for the developing world to prevent commercial transplantation. Transpl Int. 2014;27:1015-21.
- [Google Scholar]
- Paired exchange kidney donation in India: A five-year single-center experience. Int Urol Nephrol. 2012;44:1101-5.
- [Google Scholar]
- Increased rejection in living unrelated versus living related kidney transplants does not affect short-term function and survival. Transplantation. 2004;78:1030-5.
- [Google Scholar]
- Comparison of kidney paired donation transplantations with living related donor kidney transplantation: Implications for national kidney paired donation program. Ren Fail. 2013;35:504-8.
- [Google Scholar]
- Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293:1883-90.
- [Google Scholar]
- Facilitators to national kidney paired donation program. Transpl Int. 2013;26:e38-9.
- [Google Scholar]
- Exchange-donor program in renal transplantation: A single-center experience. Transplant Proc. 1999;31:344-5.
- [Google Scholar]
- The case for a living emotionally related international kidney donor exchange registry. Transplant Proc. 1986;18:5-9.
- [Google Scholar]
- Crossover renal transplantation: Hurdles to be cleared! Transplant Proc. 2001;33:811-6.
- [Google Scholar]
- Five years of single-center experience with paired kidney exchange transplantation. Transplant Proc. 2007;39:1371-5.
- [Google Scholar]
- Paired kidney donation: Outcomes, limitations, and future perspectives. Transplant Proc. 2012;44:1790-2.
- [Google Scholar]
- Transporting live donor kidneys for kidney paired donation: Initial national results. Am J Transplant. 2011;11:356-60.
- [Google Scholar]
- Successful three-way kidney paired donation with compatible pairs to increase donor pool. Ren Fail. 2014;36:447-50.
- [Google Scholar]







