Translate this page into:
Parvovirus B19 Infection as a Cause of Refractory Anemia in Kidney Transplant Recipients: A Case Series
Corresponding author: Ankur Mittal, Department of Nephrology, Medanta Institute of Kidney and Urology, Medanta—The Medicity, Gurgaon, India. E-mail: meoff2k@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hegde UN, Mittal A, Gang S, Konnur A, Patel H. Parvovirus B19 Infection as a Cause of Refractory Anemia in Kidney Transplant Recipients: A Case Series. Indian J Nephrol. doi: 10.25259/IJN_127_2024
Abstract
Background:
Kidney transplant recipients (KTRs) are at higher risk for infections, including parvovirus B19 (PVB19). This virus typically presents within the first-year posttransplant, causing anemia and potentially leading to increased morbidity and graft dysfunction.
Materials and Methods:
Charts of patients undergoing kidney transplantation between May 2013 and March 2022 were reviewed. Twenty-one patients had PVB19. Their clinical presentation, laboratory parameters, and outcomes were studied. The diagnosis of PVB19 was established by PVB19 DNA Polymerase Chain Reaction (PCR) and bone marrow examination (BME).
Results:
Prevalence of PVB19 disease was 1.9% (21/1164) with a median onset time of 39 days posttransplantation. The most frequent clinical symptoms were fatigue reported by 76% of patients, followed by fever (47%), dyspnea (23%), and myalgia (33%). All patients (100%) developed anemia, while leukopenia and thrombocytopenia were observed in 14% and 9.5% of patients, respectively. Graft dysfunction was observed in 61.9% (13/21) patients. Diagnosis was confirmed by PCR in 20 out of 21 patients. One patient had a typical viral inclusion on BME. Immunosuppression, especially antiproliferative, was reduced in all patients. Eight patients received intravenous immunoglobulin, eight received packed cell blood transfusion, and seven received erythropoietin therapy. All patients recovered, with a median time of 30 days for hemoglobin levels to normalize. One patient had graft loss secondary to graft rejection.
Conclusion:
PVB19, while uncommon, can be a significant cause of refractory anemia, particularly within the first-year posttransplant. Diagnosing PVB19 infection with PCR is crucial, and the primary treatment involves reducing immunosuppressants, especially antiproliferative agents.
Keywords
Parvovirus B19 infection
Kidney transplant
Anemia
Introduction
Parvovirus B19 (PVB19) is a common virus, belongs to the erythroparvovirus genus and Parvoviridae family with a unique ability to target red blood cells. Discovered in 1975 by Cossart et al.,1 it infects red blood cell precursors by latching onto a specific antigen (P blood group antigen) found on their surface. This widespread virus is so common that 60–90% of adults have antibodies against it, indicating past exposure.2 Infection is spread through aerosol transmission, particularly in young children manifesting as Erythema infectiosum, vertical transmission leading to Hydrops fetalis, and hematogenous transmission through blood, and blood product, and organ transplant. In immunocompetent individuals, it presents as arthropathy, transient aplastic crisis, and neurological deficit. PVB19 virus may persist in blood, bone marrow, and various tissues like liver, heart, and synovial membrane and remain dormant for several years and is reactivated during immunosuppressive state.3 Kidney transplant recipients (KTRs) are particularly susceptible to PVB19 infection due to their suppressed immune systems. Ki et al. have shown a 12% incidence of PVB19 infection among KTRs experiencing anemia, confirmed by PVB19 DNA detection in blood samples through Polymerase Chain Reaction (PCR) testing.4 This weakened immune response can lead to persistent PVB19 infection and a state of pure red cell aplasia (PRCA), characterized by a lack of red blood cell production. Notably, certain immunosuppressive medications, like antithymocyte globulin (ATG), tacrolimus, and mycophenolate mofetil, carry a higher risk of PRCA compared to azathioprine and cyclosporine.5,6 In a KTR, PVB19 disease may manifest as hepatitis, vasculitis, and myocarditis, and persistent or refractory anemia (normocytic and normochromic) unlike immunocompetent patients who present with rash or arthritis.7 PVB19 infection directly damages red blood cell precursors (erythroid series) in the bone marrow and blood, halting red blood cell production and causing existing red cells to break down. Persistent PVB19 in the kidney can trigger complications upon reducing immunosuppression. These complications include acute rejection, collapsing glomerulopathy, thrombotic microangiopathy, and chronic allograft nephropathy.8 Due to the lack of routine screening for PVB19 in KTRs, both before and after transplant, infections often go unreported. This study aimed to investigate the clinical features, lab findings, treatment approaches, and outcomes in KTRs with PVB19 infection.
Materials and Methods
It was an observational study conducted retrospectively at Muljibhai Patel Urological Hospital, Gujarat, India. Case record of all patients who underwent kidney transplantation between May 2013 and March 2022 were reviewed. Twenty-one patients were diagnosed with PVB19 infection among 73 patients being evaluated for persistent anemia. Twenty had PVB19 PCR positivity and one had typical bone marrow feature of PVB19.
Epidemiological details and clinical profiles were obtained from the hospital registry and by a telephonic conversation with the patients. Ethical approval from IEC and patient consent was obtained. Patients were analyzed for the presence of persistent anemia attributed to PVB19 infection after excluding other possible causes of anemia in posttransplant patients like nutritional deficiency (iron, Vitamin B12, or folic acid deficiency), blood loss (gastrointestinal, surgical, and trauma), and hemolysis. Primary outcomes in the form of improvement in hemoglobin concentration after treatment, occurrence of acute rejection, and graft dysfunction were analyzed. Different modalities for treatment for PVB19 infection were reviewed.
Statistical analysis
We utilized Microsoft Office Excel to analyze and visualize our data. We performed descriptive statistical analyses on both demographics and laboratory results. Numerical data, assumed to be normally distributed, was presented as average values (means) along with standard deviations to show the spread of the data. For nonnumerical data, we reported the number of occurrences (counts) and their corresponding percentages within the total population.
Results
This study identified 21 (1.9%) KTRs out of 1164 who developed PVB19 infection. All recipients had received blood group compatible live donor kidneys, and none had preexisting HIV or immunodeficiency. Two patients had cytomegalovirus (CMV) coinfection, but no other opportunistic infections were observed. The average age of the affected KTRs was 31.1 years (with a standard deviation of 10.2 years), and 18 were male. All patients presented with anemia, ranging from 3.7 to 9.4 g/dL. Peripheral blood smear analysis revealed normocytic normochromic anemia in 71.3% (15/21) of patients. Three patients had low transferrin saturation and four had positive stool tests for occult blood. Notably, the median time of PVB19 onset was 39 days posttransplant, with 76.2% (16/21) of cases occurring within the first three months (as shown in Figure 1). Bone marrow examination (BME), performed in 6 patients, revealed hypoproliferative marrow in all cases. One patient who tested negative for PVB19 PCR by blood test showed normal granulopoiesis and thrombopoiesis on BME. However, erythropoiesis was suppressed, and giant pronormoblasts with viral inclusions were observed, suggesting PVB19 infection [Figure 2].
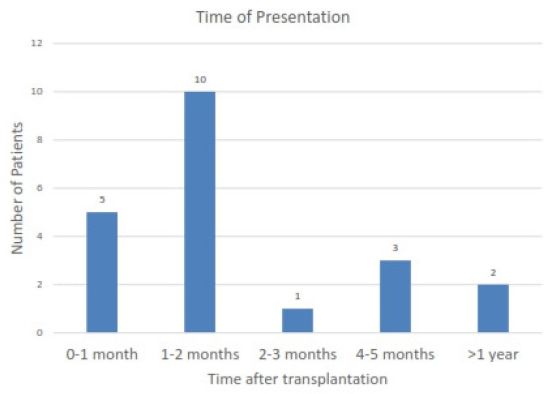
- Time of occurrence of parvovirus B19 infection after transplantation.
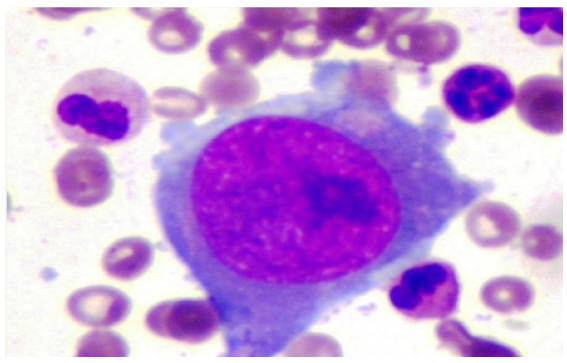
- Bone marrow picture of patients with parvovirus B19 infection.
Thirteen of twenty-one (62%) patients exhibited impaired kidney function, as shown by a rise in serum creatinine levels exceeding 30% from baseline values. Five of these 13 patients had experienced a prior episode of acute rejection, necessitating increased immunosuppression, including ATG therapy in one case. Two patients developed acute rejection after reduction in immunosuppression as the therapy for PVB19 infection. The trend of serum creatinine at presentation and on follow-up of one year is shown in Figure 3. Following PVB19 diagnosis, immunosuppression reduction, particularly antiproliferative medications, became the primary treatment approach. Additional interventions included intravenous immunoglobulin (IVIg) (varying doses and durations), red blood cell transfusions, and erythropoietin administration. Details regarding the patients’ characteristics are presented in Table 1.
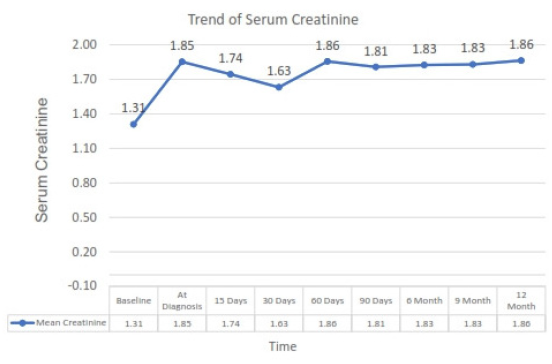
- Trend of serum creatinine level in patient at diagnosis of parvovirus B19 infection and subsequently on follow-up.
| Demographic and clinical profile | Parameters |
|---|---|
| Total number of renal transplant recipients | 1164 |
| Number of patients with Parvovirus B19 infection | 21 |
| Positive PVB19 PCR result at onset of disease | 20 |
| Bone marrow findings positive for PVB19 infection (BME Performed) | 6 (6) |
| Clinical Parameters | |
| Mean Age (Years) | 31.14 ± 10.20 |
| Gender | Male – 18; Female – 3 |
| Median time to onset | 39 days |
| Use of Induction Immunosuppression | 12 (ATG 6; Basiliximab – 6) |
| Maintenance Immunosuppression | Prednisolone, Tacrolimus, and MMF |
| Fever/Generalised weakness/Dyspnea/ Myalgia | 10 / 16 / 5 / 7 |
| Laboratory Parameters | |
| Anemia/Leukopenia/Thrombocytopenia | 21 / 3 / 2 |
| Mean Hemoglobin (mg/dL) | 6.63 ± 1.46 |
| Mean Leukocyte count (cells/mm3) | 7328 ± 2871 |
| Mean Platelets count (lakhs/mm3) | 3.34 ± 1.08 |
| Mean LDH (Units/L) | 325.12 ± 152.85 |
| Reticulocyte count (%) | 0.6 ± 0.5 |
| Treatment and Management | |
| Reduction in Immunosuppression/IVIg/ Packed cell transfusion/ Erythropoietin | 16 / 8 / 8 / 7 |
PVB19 PCR: Parvovirus B19 polymerase chain reaction; BME: bone marrow examination; LDH: lactate dehydrogenase; ATG: antithymocytic globulin; MMF: mycophenolate mofetil
All patients responded to treatment with correction of hemoglobin over the median period of 30 days. The trend of Hemoglobin at presentation and on follow-up of one year is shown in Figure 4. One patient had transient fall in hemoglobin at six months which improved on subsequent follow-up. In the remaining 20 patients, there was no recurrence of anemia up to one year after the treatment of infection.
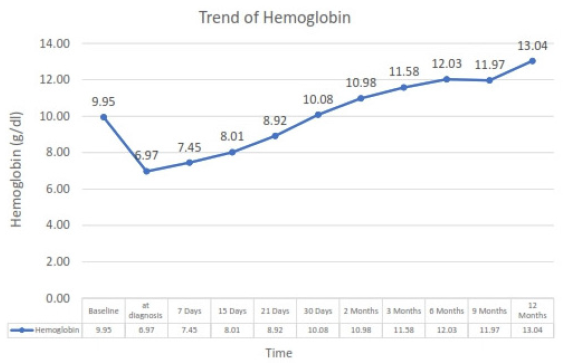
- Trend of hemoglobin level in patient at diagnosis of parvovirus B19 infection and subsequently on follow-up.
Discussion
Our study highlights the significance of PVB19 as a cause of severe and persistent anemia in KTRs and reiterates several well-known concepts. The median time for PVB19 infection to occur posttransplant was 39 days, like observations by Eid et al.8 who reported a median presentation time of 37 days. This early occurrence likely reflects the intense immunosuppression used immediately following transplantation. The study also identified cases presenting as late as one-year posttransplant, underlining the importance of PVB19 DNA testing in any KTR experiencing unexplained, persistent anemia.
Our study further shows the potential association between PVB19 infection and impaired kidney function (graft dysfunction) in KTRs. Thirteen out of the 21 patients exhibited graft dysfunction upon PVB19 diagnosis. Interestingly, five of these patients had a prior episode of acute rejection, necessitating increased immunosuppression. This raises the possibility that heightened immunosuppression might create conditions favorable for PVB19 infection. Conversely, two patients developed acute rejection following a reduction in immunosuppression, a treatment strategy used to combat PVB19. These findings highlight the delicate balance required when managing immunosuppression in KTRs with PVB19. Cavallo et al. reported elevated serum creatinine levels in 36% of patients with active PVB19 infection.9 Murer et al. proposed two possible mechanisms: thrombotic microangiopathy and direct viral infection of endothelial cells, potentially leading to increased susceptibility to acute rejection.10 While glomerulopathy has been associated with PVB19 in other studies, none of our patients presented with this complication.11 Last, Yango et al. reported a case of tubular cell damage and PVB19 DNA detection in a kidney biopsy from a patient with graft dysfunction.12 Importantly, none of the patients in our study experienced PVB19-related graft loss.
We diagnosed the infection by PCR in all but one case who needed bone marrow examination, highlighting the potential limitations of PCR testing in KTRs. Serological tests that detect IgM or IgG antibodies become less reliable in KTRs due to the inability of the patient to produce viral fighting antibodies secondary to immunosuppressive state.
Several studies, including those by Gallinella et al. and Zolnourian et al., suggest that PVB19 infection is relatively uncommon following kidney transplantation, with prevalence rates similar to the 1.8% observed in our study.13,14 A recent systematic review and meta-analysis by Thongprayoon et al. found that the incidence of detectable PVB19 DNA among KTRs can be as high as 10.3% within the first-year posttransplant, rising to 27.4% in patients with severe anemia.15
In this study, reduction in the immunosuppression, particularly the antiproliferative agent holds a primary role in the management of PVB19 infection, which will allow the body’s immune system to clear the virus by producing a specific antibody response. Kurtzman et al. in 1989 reported the first successful treatment of intravenous immunoglobulin (IVIg), but the dosage and duration of therapy remain varied.16 In our study, eight out of 21 (38%) patients received IVIg of 2gm/kg in five divided doses. Other modalities for correction of severe anemia such as packed cell transfusion, and erythropoietin administration should also be used.
In our study, the median time to the correction of hemoglobin was 30 days. Serial monitoring of hematocrit concentration and reticulocyte count is required to monitor response after reduction in immunosuppression and IVIg therapy. In this study, up to one year follow-up, there was a transient decline in hematocrit level in only one patient which improved on subsequent follow-up, which was not observed in the rest over the same duration. PVB19 viral loads were not checked in any of these patients.
Our study has some limitations. First, PVB19 testing using PCR was not performed routinely on all KTRs. It was only conducted when a patient presented with severe, persistent anemia after excluding other potential causes. Similarly, BME, which can be more sensitive for PVB19 detection, were only done in select cases and not all patients with severe anemia. Additionally, we did not perform serial quantitative PCR testing during the follow-up period. This would have provided valuable insights into the viral load dynamics and the effectiveness of treatment in clearing the virus from the body. As a single-center study, our findings may not be generalizable to the broader KTR population. A larger, multicenter study design would be necessary to obtain a more accurate estimate of PVB19 infection prevalence among KTRs.
Conclusion
PVB19 infection, although uncommon, can cause significant complications following kidney transplantation, particularly within the early posttransplant period. For any KTRs presenting with unexplained normocytic normochromic refractory anemia, with or without impaired graft function, PVB19 infection should be ruled out using DNA PCR testing or BME. The primary treatment approach involves reducing immunosuppression, especially antiproliferative medications. IVIg therapy may also be used.
Conflicts of interest
There are no conflicts of interest.
References
- Parvovirus-like particles in human sera. Lancet. 1975;305:72-3.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol. 1988;25:151-3.
- [CrossRef] [PubMed] [Google Scholar]
- Persistence of human parvovirus B19 in human tissues. Pathol Biol (Paris). 2002;50:307-16.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and clinical significance of human parvovirus B19 infection in kidney transplant recipients. Clin Transplant. 2005;19:751-5.
- [PubMed] [Google Scholar]
- Pure red cell aplasia after kidney transplantation: Parvovirus B19 culprit or coincidence? Ann Transplant. 2019;24:123-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Parvovirus B19-induced severe anemia in heart transplant recipient. Medicine (Baltimore). 2021;100:e28387.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Relapsing severe anaemia due to primary parvovirus B19 infection after renal transplantation: A case report and review of the literature. Nephrol Dial Transplant. 2007;22:3660-3.
- [CrossRef] [PubMed] [Google Scholar]
- Parvovirus B19 infection after transplantation: A review of 98 cases. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;43:40-8.
- [CrossRef] [PubMed] [Google Scholar]
- B19 virus infection in renal transplant recipients. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2003;26:361-8.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic microangiopathy associated with parvovirus b 19 infection after renal transplantation. J Am Soc Nephrol. 2000;11:1132-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of parvovirus B-19 (PV B-19) infection allows for successful kidney transplantation without disease recurrence. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2002;2:425-8.
- [CrossRef] [PubMed] [Google Scholar]
- Donor-transmitted parvovirus infection in a kidney transplant recipient presenting as pancytopenia and allograft dysfunction. Transpl Infect Dis Off J Transplant Soc. 2002;4:163-6.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence and clinical role of active parvovirus B19 infection in transplant recipients. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1999;18:811-3.
- [CrossRef] [PubMed] [Google Scholar]
- Parvovirus B19 in kidney transplant patients. Transplantation. 2000;69:2198-202.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of parvovirus B19 and anemia among kidney transplant recipients: A meta-analysis. Urol Ann. 2020;12:241-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pure red-cell aplasia of 10 years’ duration due to persistent parvovirus b19 infection and its cure with immunoglobulin therapy. N Engl J Med. 1989;321:519-23.
- [CrossRef] [PubMed] [Google Scholar]








