Translate this page into:
Perirenal Adipose Tissue: Clinical Implication and Therapeutic Interventions
Corresponding author: Ida Hosseini, Students’ Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Iran. E-mail: idahosseini.ih@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Fazeli SA, Nourollahi S, Alirezaei A, Mirhashemi S, Davarian A, Hosseini I. Perirenal Adipose Tissue: Clinical Implication and Therapeutic Interventions. Indian J Nephrol. 2024;34:573-82. doi : 10.25259/ijn_532_23
Abstract
Perirenal adipose tissue (PRAT) has been identified as an important factor in local and general homeostasis of the human body and is especially important in regulating renal and cardiovascular functions. It has also been identified as a crucial risk factor to consider in cardiovascular and renal disorders, malignancies, and various other diseases. Having a concrete idea of the effects of therapeutic interventions on the size and metabolism of the PRAT could prove highly beneficial. This review summarizes what is known about the PRAT and provides a collection of studies on the effects of therapeutic interventions on PRAT and its related diseases. We used papers written on a variety of subjects, mainly concerning adipose tissue and the effects of therapeutic procedures on it. Our main challenge was to excerpt the information specifically related to the PRAT in these papers. These effects vary greatly, from an increase or decrease in mass or size of the PRAT to changes in metabolism and drug residue accumulation. The current studies often fail to consider PRAT as an individual subject of research and only examine the adipose tissue of the entire body as a whole. This leads us to believe this field could benefit greatly from further research.
Keywords
Perirenal adipose tissue
Adipose tissue
Cardiovascular diseases
Obesity
Renal pathology
Interventions
Introduction
Adipose tissue typically represents 20–25% of the body weight in healthy adults. Its functions are: storing neutral fats (mainly triglycerides), acting as regulators of the body’s energy metabolism, thermogenesis, and protection against trauma. Excessive adipose tissue leads to obesity, which can be treated by exercising, a healthy diet, and therapy.
The perirenal adipose tissue (PRAT) surrounds each kidney and is located between the kidney’s capsule and fascia. PRAT’s endocrine and paracrine activities play a significant role in local and systemic homeostasis. In addition, PRAT can affect the pathology of multiple diseases, which will be discussed later in this paper.
Although the anatomy and morpho-physiology of the PRAT itself and its implications in clinical complications such as whether abnormalities of the PRAT should be considered as risk factors in cardiovascular and renal diseases have been studied in detail, the effects that therapeutic interventions have on its size and anatomy are relatively new fields of study and have much room for extensive research.
This study aims to compile the existing research on these subjects to identify better the fields that require further research.
Materials and Methods
We review and summarize the current information about the PRAT, its role in causing diseases, and ways to prevent its adverse effects. The keywords “Perirenal adipose tissue; Adipose tissue; Cardiovascular diseases; Obesity; Renal pathology; Interventions” were searched in various databases, including ISI Web Of Science, PubMed, Google Scholar, and Scopus. Related English articles published till 2022 were reviewed. Because of this field’s ever-changing knowledge, the focus was mainly on papers after 2010.
The adipose tissue
There are three types of adipose tissue: white, brown, and beige. Each has specific morphological features. What they all have in common are adipocytes, preadipocytes, pericytes, inflammatory immune cells, blood vessels, and nerve endings.1
The human body fat is mainly made of white adipose tissue (WAT), found in subcutaneous and visceral areas [such as around the gonads, epicardium, retroperitoneum, mesentery, omentum, and the kidneys (perirenal)].2,3 WAT stores energy in the form of triglycerides, which then can be decomposed into free fatty acids and glycerol during the lack of food.4 WAT has also been associated with obesity and its relative complications.5
In adults, the brown adipose tissue (BAT) exists only in scattered areas around the kidneys, adrenal glands, aorta, and mediastinum. Its brown color is due to blood vessels and mitochondria filled with cytochrome pigmentations. BAT burns fatty acids which lead to heat production. Thermogenin or uncoupling protein-1 (UCP-1) is known to mediate this heat-producing process.6 Contrary to prior knowledge about BAT, it is known today that this tissue is not only active in babies but has an essential role in adults as well. Some studies have shown that BAT plays a pivotal rule in thermogenesis, clearance of triglyceride and glucose disposal, secreting adipokines, with a distinct inflammatory characteristics in comparison to WAT as well as thermogenesis.7
A new type of adipocyte has been discovered recently, which shows different gene expressions from white or brown adipocytes. These brown-like cells found among WAT, especially in the inguinal area, were named beige or brite “(‘brown in white’ adipocytes or inducible brown adipocytes).”5
WAT structures can gain BAT-like features. This process is called browning and is done in response to appropriate stimuli. The primary regulators of BAT development are PRDM16, PPARγ, and PGC-1α. WAT adipocytes take on characteristics of BAT like expressing UCP-1, multilocular lipid, and many mitochondria.8 This reverses their role as energy-storages to energy-releasing entities. Some regulators such as Irisin and FGF21 are secreted from muscles due to exercising.9-11 FGF21 is a hormone mainly produced in the liver, identified as a regulator for the browning process by affecting PGC-1α.12 It can increase by being exposed to cold.13 Some characteristics of white and brown adipose tissues are mentioned in Table 1.14
| White adipose tissue | Brown adipose tissue | |
|---|---|---|
| Morphology |
Unilocular adipocytes Fewer mitochondria compared to BAT |
Multilocular adipocytes More mitochondria compared to WAT |
| Function | Energy storage, food supply during fasting | Thermogenesis, inflammatory functions |
| Main origin | Myf5-negative cells | Myf5-positive cells |
| Association with diseases | Increased risk of obesity-related diseases | Decreased risk of obesity-related diseases |
| Proportion in adults vs. infants | Higher in adults | Higher in infants |
| Areas in the body | Under the skin – around the gonads, epicardium, retroperitoneum, mesentery, omentum, and kidneys | Around the kidneys, adrenal glands, aorta, and mediastinum |
BAT: brown adipose tissue, WAT: white adipose tissue
The PRAT
There are three sections of visceral adipose tissue around each kidney. PRAT is the adipose tissue surrounding the kidney and the adrenal glands, situated between the renal capsule and the renal fascia in the retroperitoneum. The renal sinus fat is the adipose tissue situated in the medial border of the kidneys, encompassing the renal blood vessels, nerves, and lymphatic vessels, and could be considered a part of the body’s perivascular adipose tissue and has a role in controlling blood pressure.15,16 Lastly, the paranephric adipose tissue surrounds the PRAT, being located outside of the renal fascia (Gerota’s fascia).8,17
PRAT has the exact fetal origin as typical visceral fat.18 However, it has unique characteristics: a complete blood supply system, lymph drainage, and innervation.19-21 This makes it similar to internal organs rather than other connective tissues. The abdominal aorta’s branches, including inferior adrenal, gonadal, left colic, renal, and lumbar, generate an anastomosing capillary network for the PRAT.22,23 Its nerves originate from the celiac, superior mesenteric, ipsilateral inferior mesenteric, adrenal, aortic, renal, ovarian, and testicular nerves, and the ipsilateral sympathetic chain ganglia.24 Perirenal lymphatic vessels end in subcapsular vessels and para-aortic nodes.25 These features form the connection between the kidney and PRAT.26 In addition, compared to subcutaneous adipose tissues, the proportion of saturated fatty acids is higher in PRAT.27
Studies on PRAT adipogenesis have shown that preadipocytes in the perirenal area do not express endothelial markers such as CD31 or hematopoietic stem cell markers like CD45, but are positive for CD90 and CD166.28
Morphologically speaking, PRAT consists of a combination of WAT and BAT, along with a combination of mesanchymal stem cells, inflammatory stem cells, preadipocytes, capillaries, and nerves.8 In fetuses and babies younger than 11 months, BAT forms the majority of PRAT while WAT only exists as a thin outer layer;26 that is, until the brown adipocytes start developing into unilocular white adipocytes, leading to brown cells only existing as small cellular clusters in the WAT. At this time, about 40% of PRAT has a typical brown adipocyte morphology, while about 30% expressed UCP-1.29 Furthermore, in adults, these brown adipocytes are mostly in a dormant state and active brown adipocytes are only seen in areas with a high number of sympathetic nerve endings.28 SPARC and CLSTN3 have been known for the dormant and multilocular BAT states, respectively.28
The adipocytes of the PRAT have a significant role in homeostasis due to their role in the secretion of various adipokines or cytokines (adiponectin, leptin, resistin, tumor necrosis factor-α (TNF-α), visfatin, interleukin-6 (IL-6), and IL-1β), which are regulators for the kidneys.8,30 Leptin and adiponectin are also known to link obesity to high blood pressure and chronic kidney diseases (CKD).31
PRAT has a large number of mesenchymal stem cells (MSCs) and like other MSCs found in other fat tissue depots have the potential to differentiate into adipocytes, or otherwise, osteogenic, chondrogenic, and epithelial cells. Recent studies have shown that in the presence of inflammatory factors produced by the body’s immune system, such as IL-1β, IFNγ, and TNF-α, these MSCs express an immunoregulatory response. Moreover, secretion of IL-6 and IL-8 by these MSCs could be stimulated by TNF-α which is associated to enhanced angiogenesis in experimental model. The immunosuppressive features of the MSCs hosted in PRAT can be enhanced by IL-6, TNF-α, and IFNγ in vitro. These observations may suggest a possibility of using MSCs in therapeutic interventions for inflammatory disease, tissue injuries, and cardiovascular and renal diseases.3
Quantifying PRAT’s size is done by ultrasound, magnetic resonance imaging, and computer tomography;26 the second and third options are considered unsuitable due to being expensive and time-consuming and exposing the patient to radiation. PRAT thickness in healthy people is 7.95 mm on average.32 And, amounts greater than 10 mm (or area greater than 100 cm2) are considered fat accumulation.33
Perirenal fat has weak relations with BMI.32 Moreover, the PUFT measure (the thickness of perirenal fat, evaluated by ultrasound method) correlates more with total and visceral fat.34
PRAT in the population
Men tend to have more significant perirenal fat than women.35 However, PRAT in females has a higher potential to gain BAT-properties than males. The degree of the browning depends on sex-specific and environmental factors such as temperature (6). It was shown in a study that the presence of Y-chromosome suppresses the expression of UCP-1.36
Analyzing the UCP-1 gene expression revealed significant variations of the PRAT in the population. Both dormant and active BATs were detected in several individuals. This analysis identified 54 overexpressed genes in UCP-expressing cells. A set of genes with high correlation to UCP-1 had been revealed by Real-time PCR analysis of BAT. Some genes of transcription factor i.e. PRDM16, PGC1a, RXRc were highly correlated to each other.
Brown adipocytes showed nuclear immunoreactivity of RXRc. Brown adipogenesis was associated with increased gene expression of RXRc in human stem cells.37
PRAT’s association with other diseases
Obesity is associated with cardiovascular disease (CVD) and CKD.26 Moreover, PRAT may be considered as a connection between these two. These complications, among others, will be discussed in the following.
Renal diseases
PRAT has a closer relation with renal disease and CVD than other visceral fat. Furthermore, it is considered a risk factor for CKD making the risk 2–3 times greater.38 It may even connect CVD and CKD.26 Figure 1 demonstrates some possible pathways of PRAT causing CKD.
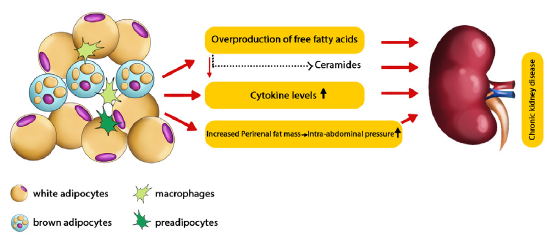
- Possible pathways of perirenal adipose tissue causing chronic kidney disease.48
Para- and perirenal fat thickness can be considered predictors of renal dysfunction in type-2 diabetes, and being an essential piece of the inter-individual variability of glomerular filtration rate (GFR), renal resistance index, and uricemia.39
High PUFT is associated with a low GFR34 and shows the risk of developing arterial hypertension and chronic renal diseases.25
Obesity can cause proteinuria and glomerulopathy in nonproteinuria patients with renal dysfunction.40-42 PRAT is larger in obese patients with albuminuria,23 which is caused by the overproduction of free fatty acids (FFA).32,43
The amount of FFAs in renal and jugular venous blood was compared, and the results showed lower amounts of FFAs in the jugular vein, which indicated that the overproduced FFAs acted as kidney-damaging factors. This impact is caused either directly or indirectly via mediators.44 These FFAs also cause lipotoxicity in kidneys (resulting in chronic inflammations) by FFA metabolites, such as ceramides.43
The inflammatory result of FFAs can lead to an imprudent amount of cytokine secretion,45,46 causing NO imbalance in the endothelium and renal arterial dysfunction.47
Due to the fascia surrounding PRAT, an increase in perirenal fat accumulation and intra-abdominal pressure can compress the kidneys, increasing sodium absorption and blood pressure. It also leads to increased hydrostatic pressure of renal interstitial fluid and reduced flow rates of renal blood and tubular fluid.41,48
Radiological renal positioning49 and staging clear cell renal carcinoma (ccRCC)50 can be done by evaluating the PRAT. PRAT’s measurement, tumor location, and age are notable predictors of clear cell renal carcinoma’s histopathology.51
Fat stranding is an increase in attenuation, which can be seen in infections, inflammations, malignancies, or traumas.52 Perinephric stranding is seen as the appearance of edema within the PRAT on CT or MRI.
Some degrees of symmetric bilateral perinephric stranding are common in older people. However, the asymmetric or unilateral form is an important sign of kidney inflammation or acute obstruction (like acute pyelonephritis and ureteric calculi). An association with bladder outlet obstruction has also been suggested.53
A study on 174 patients established a relation between PRAT size and ccRCC, caused by the anatomical relations between these two structures.54 Another study has concluded that PRAT’s high amounts of UCP-1 production make a bad prognosis for clear cell renal carcinoma. This study also compares PRAT’s characteristics in healthy people and clear cell renal carcinoma patients, showing the following results: A reduction in HOXC8 and HOXC9 genes expression and an unchanged expression in TBX1, TMEM26, CD137, ADIPQ, and LEP. These results indicate a browning process in clear cell renal carcinoma patients’ PRAT.55
Cardiovascular diseases
Increased PRAT size is associated with a high risk for cardiovascular and metabolic complications.56,25
Many studies have shown a correlation between CKD and CVD; therefore, a connection between albuminuria and CVD can be assumed.57-59
Metabolic complications caused by PRAT such as diabetes or dyslipidemia tend to increase the risk of CVDs indirectly.26
This fat layer may relate to epicardial fat because of the common features it has with mesothelial layers of visceral organs enriched in WAT progenitors producing adipocytes.60
Fat storage in the renal sinus has been related to acute and chronic hypertension.61 A study has shown that fat storage in the renal hilum and PRAT compresses the lymphatic and venous vessels and the ureters, resulting in the activation of the renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS) activity; this will increase the hydrostatic pressure and lead to hypertension, resistance to insulin, and atherosclerosis.56,62,63
Adipose tissue nerves affect the SNS by a negative feedback loop (reflex). PRAT increases the renal sympathetic outflow by this means, causing higher arterial blood pressure.64 A study has suggested that PRAT directly regulates the cardiovascular system. This was indicated as a result of leptin injection in PRAT, which activated the reflex without increasing the activator substances.65
However, endothelial injury is known to be the initiator for CVD,26 which is caused by the same processes described in the subsection “Renal diseases.”
Other complications
Greater PRAT thickness measures tend to relate to dysfunction in the regulation of glucose homeostasis, such as impaired levels of fasting glucose increased insulin resistance and high triglyceride and uric acid levels in CKD patients.60,66
PRAT surface area, measured by CT scans, is used for preoperative assessments. Its median is calculated to be 24 cm2. A study showed that patients with bigger PRAT areas may have some risk by undergoing colon cancer surgery. Patients with PRAT ≥ 40 cm2 had longer operations, bled more during the operation, had to undergo the operation twice, and suffered from surgical and nonsurgical complications. However, they didn’t differ from PRAT < 40 cm2 patients in terms of the need for intensive care or the time they stayed in the hospital.67 However, another study concluded that the PRAT area is not an important indicator of negative results in rectum cancer patients who undergo laparoscopic surgery.68
Obesity has been known to be an important risk factor for cancer. Malfunction of the adipose tissue is a source for excessive amounts of cytokines, growth factors, and adipokines that link to tumor cell growth in these patients.69-71
The factors involved in tumor progression induced by PRAT are high amounts of UCP-1, low amounts of HOXC8&9 expression, browning, and cachexia as a result and adipokines and cytokines produced by the dysfunctioning PRAT.55,48
Older male patients with symptoms in the lower urinary tract tend to show severe PRAT stranding in their CT scans. The stranding’s severity is related to the level of bladder outlet obstruction and diminished kidney function.53
Peri- and pararenal fat thickness is a marker for the presence and severity of a fatty liver.33
Occlusive carotid artery disease is caused by atherosclerotic plaques that narrow or completely block the artery’s blood flow and increase the risk of stroke. HIV infection can lead to this, and the ophthalmic artery resistance index (OARI) is an index of this disease in HIV-1 positive patients. A study indicated that assessing PRAT’s thickness via ultrasound (PRFT) might indicate endothelial damage (particularly of the vascular area of the eyes) in these patients.72
In research about approaches to pediatric simple renal cysts (which included the PRAT wadding technique), the cysts were filled with perirenal fat tissue after deroofing and seemed to reduce recurrence.73
PRAT’s pathologies
Perirenal aortic atherosclerosis starts in the early stages of life. Furthermore, it has similar plaque morphologies to coronary atherosclerosis. However, its macrophage content tends to differ site-specifically.74
Nontraumatic perirenal hematoma is an uncommon condition in which severe cases can lead to shock. Bevacizumab treatment is known to be related to many bleeding complications; therefore, a study suggested an association between PRAT hematoma and Bevacizumab.75
Adherent perirenal fat (APF) is defined as an inflammatory fat that surrounds the kidney. Measuring PRAT’s thickness via CT scan can predict APF. This condition challenges kidney surgery by making the dissection difficult, which increases the duration of PRAT dissection and surgery.76 Figure 2 summarizes PRAT’s association with other diseases.
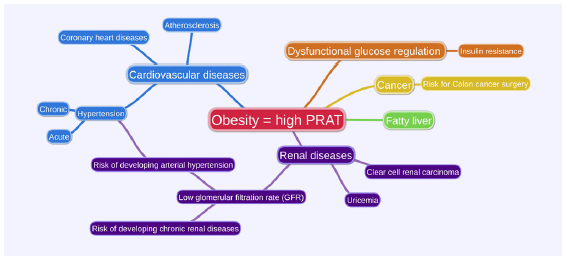
- Perirenal adipose tissue (PRAT)’s association with other diseases.
Therapeutic interventions
As several studies have concluded, high PRAT mass leads to CVD, CKD, and other disorders. A series of materials, diets, diseases, and interventions which decrease PRAT mass are reviewed and categorized in the following sections and summarized in Table 2.
| Pharmaceuticals | Herbals/others | Complementary | Lifestyle |
| Olanzapine | Kurozo | Acupuncture at the Fengiong Acupoint | Dining twice a day |
| Beta-3 adrenergic receptor agonists | Green tea | Electroacupuncture | Low-calorie diet |
| Telmisartan | Lysimachiafoenum-graecum | Cold exposure | |
| Betulonic acid | Palmiwon | ||
| Evodiamine | C. Tumida | ||
| Quasimodo | Nelumbiris semen | ||
| Fructus Schisandrae Aqueous | |||
| Fish oil |
Pharmaceuticals
Pharmaceuticals which were not proven to have any decreasing effect
Animals chronically treated with olanzapine (±6.5 mg/kg/day, orally administered) had an increase in serum leptin and PRAT weight.77
Statins are used for treating hypercholesterolemia. A study scrutinized its effect on body fat and concluded that it does not decrease intra-abdominal fat (including PRAT).78
The thiazolidinediones are a class of compounds that lower blood glucose in diabetic rodent models. A study examined the effect of thiazolidinedione PIO (pioglitazone) on UCP mRNAs in brown PRAT of mice. The treatment caused the BAT, UCP, and aP2 mRNAs to increase in all models and also decreased the serum glucose and insulin levels, 2 and 5.5 times, respectively, in some of the mice (the ob/ob).79
Treatment with PIO has shown to increase bromodeoxyuridine (BrdU) levels in subcutaneous mature adipocytes (not visceral fat including PRAT) and increase the number of small adipocytes and proliferation in these areas. However, visceral adipocytes absorbed BrdU more actively than subcutaneous areas.80
Combination therapy with canagliflozin and pioglitazone has shown to reduce high levels of glucose and insulin in obese, type-2 diabetes patients. The addition of canagliflozin prevents glucotoxicity and reduces the weight gain caused by PIO; therefore, it improves insulin sensitivity.81
A study worked on the effects of liraglutide and PIO on mice. Perirenal, inguinal, and epididymal fats and body weight showed to be larger and more in PIO-treated mice rather than the mice which were treated with a vehicle. However, combining the two drugs caused the most weight gain amongst all models because of the glucosuria reduction causing improved balance in energy.82
Adipose tissues secrete leptin which can be used to indicate the total mass of adipose tissues along with the PRAT mass.83 Anthocyanins (ACNs) from many ingredients have proven to reduce obesity in some animals. A study has compared the outcomes of purified black raspberry (BRB) ACNs and freeze-dried whole BRB feeding on developing obesity. But there was no decrease in serum leptin (related to the amount of adipose tissues) in purified ACN-treated models (which had a high-fat diet).84
Pharmaceuticals with a decreasing effect
Mice fed with a standard or high-fat diet were given intraperitoneal Standardized Quassinoids-enriched fraction (SQEL) at 5 mg/kg and 10 mg/kg doses concurrently for 12 weeks. The results indicated that SQEL suppresses weight gain and decreases PRAT mass.85
Erdheim-Chester disease (ECD) is a rare and fatal multi-system disease of adulthood. It is characterized by excessive proliferation and accumulation of histiocytes, a type of macrophage. Its most prominent cause of death is cardiovascular complications.86 In 30% of cases, there is renal involvement due to fibrotic tissue being deposited in the PRAT. For this reason, analyzing the PRAT for infiltrates is the gold standard for ECD’s diagnosis. A study on mice showed that oral administration of evodiamine, an alkaloidal chemical compound extracted from Evodia fruits (Evodia rutaecarpa), at 0.03% and 0.02% of the diet for days resulted in a considerable reduction in the PRAT mass. Furthermore, increased lipolysis in the PRAT and specific GDP binding in BAT mitochondria (considered a biological index of increased heat production) were observed.87
Betulinic acid is a pentacyclic triterpenoid that has been a research target for anti-cancer usage. In research on CD-1 mice, the distribution of betulinic acid in PRAT after 24 hours of the intraperitoneal administration was the highest among other targeted tissues.88
Telmisartan is an oral medication for treating high blood pressure. A study on this medicine’s effect on a high-fat diet has shown improvement in insulin resistance and dyslipidemia, and due to an increase in the expression of adiponectin mRNA, elevated serum adiponectin levels. Moreover, it caused a reduced PRAT mass (which would typically increase due to a high-fat diet). This study suggested a connection between PRAT and hypertension (caused by high fat intake), and Telmisartan has been suggested as a candidate choice for its treatment.89
Complementary and herbal medicine
In a study on rats, Kurozu concentrated liquid (KCL) was orally administered to them at a 100 mg/kg dose. It caused a decrease in the size of adipocytes by preventing fat absorption in the gastrointestinal tract and reducing the expression of PPARγ and aP2 mRNA in the fat cells. This resulted in a higher number of small adipocytes in the PRAT and, consequently, a reduction in PRAT size.90
Supplementation of green tea extract (GTE) significantly reduced perirenal and total WAT weights.91
Dose-dependent treatment of high-fat diet (HFD) mice with lysimachia foenum-graecum extract (LFE) showed inhibition in adipogenesis and lipid metabolism and resulted in the reduction of the weight of WAT. This marks LFE as a therapeutic option for obesity.92
Palmiwon reduces perirenal fat accumulation by preventing lipid accumulation as a whole and, therefore, may be considered as a therapeutic option for hyperlipidemia.93
Dietary ingestion of immature C. tumida peels suppressed body weight gain following decreased perirenal fat weights.94
Nelumbinis semen (NS) powder supplementation has been shown to reduce the perirenal fat weights significantly.95
A study showed lower PRAT amounts in sorghum extract–administrated mice rather than the control group (which both were on a high-fat diet), and a higher PPAR-γ and adiponectin expression. However, TNF-α was significantly expressed less in these mice.96
Feeding mice on agaropectin-derived oligosaccharides (SAOs) in a study has resulted in a decrease of body weight and adiposity index. SAOs also reduced lipid accumulation in the liver, PRAT, and epididymal fat tissues.97
In a study, Fructus Schisandrae aqueous extract (FSE) treatment alone reduced the weight gain and increase in fat pad mass (including PRAT) caused by dietary habits, which makes FSE a potential preventive substance for controlling weight.98
Electroacupuncture intervention (a form of acupuncture in which a small electric flow passes between two needles) has been proven to reduce weight. However, it has different amounts of effects based on gender. The effect on perirenal fat weight is more significant in male obese rats.99
Preventive measures
By having a healthy lifestyle, regular exercise and a controlled diet, obesity can be prevented or treated.
Restricting calories, dining twice a day, having fish oil included in the diet, and etc., are some examples of dieting that can help reduce PRAT.100-102
A study on rats had them develop a metabolic syndrome by feeding them high-fat-salt food, and then some groups had to run on a treadmill. In conclusion, this aerobic exercise increased PPARαmRNA and protein expression in the myocardium and LDL in the blood. It also reduced body mass and visceral fat mass (and PRAT’s weight).103
In the case of preventive treatment, research on the effect of bilateral needling of the Fenglong acupoint plus having a high-fat diet in rats has shown that the measurement of PRAT in rats with Fenglonf acupoint has been lower than rats with a high-fat diet (and no acupuncture). In conclusion, this preventive treatment (acupuncture at the Fenglong acupoint) can drastically prevent obesity in rats and help improve abnormal fat metabolism. This can be related to the improvement of leptin resistance and PPAR-γ expression in the fat tissue. This type of intervention can also fall under the complementary medicine type.104
Other
Since the activation of BAT can increase glucose uptake and energy usage105 and considering the correlation of WAT with diseases, reactivating the dormant BAT into active BAT has been a suggestive treatment for increasing PRAT. This can be done by exposure to cold or stimulating the β3-adrenoreceptors.29,106
lncRNAs are known to affect a process that is the opposite of browning, transforming BAT to WAT. Twenty-one thousand two hundred and thirty-two kinds of lncRNAs were found in goat PRAT’s genome, of which 548 were identified in the mentioned process. Analysis of the lncRNA and mRNAs has shown that these genes may regulate the BAT to WAT process by affecting the AMPK signaling pathways, fatty acid metabolism, and cell respiratory chains.107
Heme-oxygenase (HO) reduces PRAT and mediators including (MIP-1α), (ET-1), TNF-α, IL-6, and IL-1β.108
In the past decade, PRAT’s inherent anatomical and morphophysiological features have been researched sufficiently. It has been determined that the size and area of PRAT have significant direct and indirect effects on the pathologic path, risk, and prognosis of numerous cardiovascular, renal, and metabolic diseases and various malignancies. Different therapeutic interventions have varying effects on the anatomical and metabolic attributes of PRAT. Some cause an increase in mass and size, while some reduce it. Some pharmaceutical options even change the metabolic traits of the PRAT.
Due to the considerable ramifications that PRAT causes on the disorders mentioned above and their implications on their prognoses, having a good understanding of these effects is critical and could assist in making better therapeutic decisions. The research conducted on this matter has been scarce and often indirect, with the studies being primarily on the entire body’s adipose tissue instead of directly focusing on the PRAT. Further research is needed to fully understand the effects of various therapeutic interventions on the PRAT as an individual part of the body’s adipose tissue.
Conflicts of interest
There are no conflicts of interest.
References
- PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front Physiol. 2018;9:70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Deciphering adipose tissue heterogeneity. Ann N Y Acad Sci. 2018;1411:5-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Isolation, characterization, differentiation and immunomodulatory capacity of mesenchymal stromal/stem cells from human perirenal adipose tissue. Cells. 2019;8:1346.
- [CrossRef] [PubMed] [Google Scholar]
- Uncoupling protein 1: A short-circuit in the chemiosmotic process. J Bioenerg Biomembr. 2008;40:457.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose tissue: White adipose tissue structure and function. In: Caballero B, Finglas PM, Toldrá F, eds. Encyclopedia of Food and Health. Oxford: Academic Press; 2016. p. :35-42.
- [Google Scholar]
- Women have more potential to induce browning of perirenal adipose tissue than men. Obesity. 2015;23:1671-9.
- [CrossRef] [PubMed] [Google Scholar]
- Brown adipose tissue. Adipocyte. 2012;1:13-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci Rep. 2013;33:e00065.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Investig. 2014;119:37-25.
- [CrossRef] [PubMed] [Google Scholar]
- FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Brown and beige fat: Development, function and therapeutic potential. Nat Med. 2013;19:1252-63.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of FGFs as Adipokines in Adipose Tissue Development, Remodeling, and Metabolism. Front Endocrinol (Lausanne). 2014;5:18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Perirenal fat: A unique fat pad and potential target for cardiovascular disease. Angiology. 2019;70:584-93.
- [CrossRef] [PubMed] [Google Scholar]
- Renal sinus fat and renal hemodynamics: A cross-sectional analysis. MAGMA. 2020;33:73-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal and renal sinus fat volumes as quantified by magnetic resonance imaging in subjects with prediabetes, diabetes, and normal glucose tolerance. PLOS ONE. 2020;15:e0216635.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The relationship between perirenal fat thickness and reduced glomerular filtration rate in patients with type 2 diabetes. J Diabetes Res. 2020;2020:6076145.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anatomical and enzyme histochemical differentiation of adipose tissue. Int J Obes. 1985;9(Suppl 1):1-6.
- [PubMed] [Google Scholar]
- Fetal topographical anatomy of the upper abdominal lymphatics: Its specific features in comparison with other abdominopelvic regions. Anat Rec (Hoboken). 2012;295:91-104.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of ganglionic sympathetic neurons supplying the subcutaneous, perirenal and mesentery fat tissue depots in the pig. Acta Neurobiol Exp (Wars). 2002;62:227-34.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of Dietary Fat on the Lipid Composition of Perirenal Adipose Tissue in Rats. Annals of Nutrition and Metabolism. 1990;34:327-32.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and blood supply of the coeliac-superior mesenteric ganglion complex of the rat. Anat Embryol (Berl). 1981;162:353-62.
- [CrossRef] [PubMed] [Google Scholar]
- Peri-renal adipose inflammation contributes to renal dysfunction in a non-obese prediabetic rat model: Role of anti-diabetic drugs. Biochem Pharmacol. 2021;186:114491.
- [CrossRef] [PubMed] [Google Scholar]
- Morbid obesity and hypertension: The role of perirenal fat. J Clin Hypertens (Greenwich). 2018;20:1430-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel insight into perirenal adipose tissue: A neglected adipose depot linking cardiovascular and chronic kidney disease. World J Diabetes. 2020;11:115.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Fatty acid composition of adipose tissue in humans: Differences between subcutaneous sites. Am J Clin Nutr. 1989;50:288-91.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol Metab. 2019;24:30-43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem. 2020;76:185-92.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing the serum levels of Adipocytokines in the renal transplant recipients and healthy individuals: A case-control study. Iranian Red Crescent Medical Journal. 2018;20:3.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic evaluation of para- and perirenal fat thickness is an independent predictor of early kidney damage in obese patients. Int Urol Nephrol. 2013;45:1589-95.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic assessment of fatty liver infiltration using the measurement of para-and perirenal fat thickness. J Clin Ultrasound. 2010;38:470-4.
- [CrossRef] [PubMed] [Google Scholar]
- Para-perirenal distribution of body fat is associated with reduced glomerular filtration rate regardless of other indices of adiposity in hypertensive patients. J Clin Hypertens (Greenwich). 2018;20:1438-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gender differences in subcutaneous and perirenal fat distribution. Surgical and radiologic anatomy. 2010;32:879-82.
- [CrossRef] [PubMed] [Google Scholar]
- The number of x chromosomes causes sex differences in adiposity in mice. PLoS genetics. 2012;8:e1002709.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Characterization of brown adipose tissue in the human perirenal depot. Obesity. 2014;22:1830-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension. 2011;58:784-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Para-and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011;26:892-8.
- [CrossRef] [PubMed] [Google Scholar]
- Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol. 2004;164:385-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney int. 2006;70:1636-41.
- [CrossRef] [PubMed] [Google Scholar]
- Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant. 2009;24:3732-8.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of biliverdin reductase-A promotes lipid accumulation and lipotoxicity in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2018;315:F323-F31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Perirenal fat associated with microalbuminuria in obese rats. Int Urol Nephrol. 2014;46:839-45.
- [CrossRef] [PubMed] [Google Scholar]
- Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28:S58-S65.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal peri-organ or intra-organ fat (APIFat) deposition: An underestimated predictor of vascular risk? Curr Vasc Pharmacol. 2016;14:432-41.
- [CrossRef] [PubMed] [Google Scholar]
- Perirenal fat promotes renal arterial endothelial dysfunction in obese swine through tumor necrosis factor-α. J Urol. 2016;195:1152-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Perirenaladipose tissue—current knowledge and future opportunities. J Clin Med. 2021;10:1291.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Spontaneous subcapsular and perirenal hematomas. Radiology. 1989;172:601-2.
- [CrossRef] [PubMed] [Google Scholar]
- Perirenal fat invasion on renal cell carcinoma: Evaluation with multidetector computed tomography–Multivariate analysis. J Comput Assist Tomogr. 2013;37:450-7.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of perirenal fat as a predictor of cT1a renal cortical neoplasm histopathology and surgical outcomes. J Endourol. 2012;26:911-6.
- [CrossRef] [PubMed] [Google Scholar]
- Fat stranding (CT) Radiopaedia.org2020 Available from: https://doi.org/10.53347/rID-47438.
- Perirenal fat stranding on CT: Is there an association with bladder outlet obstruction? Br J Radiol. 2016;89:20160195.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Huang H, Chen S, Li W, Wu X, Xing J, eds. High perirenal fat thickness predicts a poor progression-free survival in patients with localized clear cell renal cell carcinoma. Urologic Oncology:Seminars and Original Investigations; 2018. Elsevier
- Increased UCP1 expression in the perirenal adipose tissue of patients with renal cell carcinoma. Oncol Rep. 2019;42:1972-8.0.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Para-and perirenal ultrasonographic fat thickness is associated with 24-hours mean diastolic blood pressure levels in overweight and obese subjects. BMC Cardiovasc Disord. 2015;15:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy:the LIFE study. J Hypertens. 2002;203:12-405.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of renal and vascular end stage disease (PREVEND) study group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777-82.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of microalbuminuria and its associated cardiovascular risk: German and Swiss results of the recent global i-SEARCH survey. Swiss Med Wkly. 2009;139:473-80.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship of para-and perirenal fat and epicardial fat with metabolic parameters in overweight and obese subjects. Eat Weight Disord. 2019;24.1:72-67.
- [CrossRef] [PubMed] [Google Scholar]
- Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ultrasound-assessed perirenal fat is related to increased ophthalmic artery resistance index in HIV-1 patients. Cardiovas Ultrasound. 2010;8:24.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity, kidney dysfunction, and inflammation: Interactions in hypertension. Cardiovasc Res. 2021;117:1859-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol (1985). 2012;112:1008-14.
- [CrossRef] [PubMed] [Google Scholar]
- Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci Lett. 2000;293:107-10.
- [CrossRef] [PubMed] [Google Scholar]
- Perirenal fat thickness is associated with metabolic risk factors in patients with chronic kidney disease. Kidney Res Clin Pract. 2019;38:365.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Perirenal fat surface area as a risk factor for perioperative difficulties and 30-day postoperative complications in elective colon cancer surgery. Colorectal Dis. 2018;20:1078-87.
- [CrossRef] [PubMed] [Google Scholar]
- Increased perirenal fat area is not associated with adverse outcomes after laparoscopic total mesorectal excision for rectal cancer. Langenbecks Arch Surg. 2017;402:1205-11.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121-35.
- [CrossRef] [PubMed] [Google Scholar]
- Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498-503.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ultrasound-assessed perirenal fat is related to increased ophthalmic artery resistance index in HIV-1 patients. Cardiovascular Ultrasound. 2010;18:9-1.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic or robotic deroofing guided by indocyanine green fluorescence and perirenal fat tissue wadding technique of pediatric simple renal cysts. J Laparoendosc Adv Surg Tech A. 2020;30:471-6.
- [CrossRef] [PubMed] [Google Scholar]
- The natural history of aortic atherosclerosis: A systematic histopathological evaluation of the peri-renal region. Atherosclerosis. 2010;210:100-6.
- [CrossRef] [PubMed] [Google Scholar]
- Perirenal hematoma associated with bevacizumab treatment. Invest New Drugs. 2012;30:808-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pathology associated with adherent perirenal fat and its clinical effect. Int J Clin Pract. 2021;75:e14518.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic treatment with olanzapine increases adiposity by changing fuel substrate and causes desensitization of the acute metabolic side effects. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:185-95.
- [CrossRef] [PubMed] [Google Scholar]
- Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J Physiol Pharmacol. 2013;64:281-8.
- [PubMed] [Google Scholar]
- Induction of uncoupling protein in brown adipose tissue: Synergy between norepinephrine and pioglitazone, an insulin-sensitizing agent. Biochem Pharmacol. 1996;52:693-701.
- [CrossRef] [PubMed] [Google Scholar]
- Pioglitazone enhances small-sized adipocyte proliferation in subcutaneous adipose tissue. Endocr J. 2012;59:1107-14.
- [CrossRef] [PubMed] [Google Scholar]
- Beneficial effects of canagliflozin in combination with pioglitazone on insulin sensitivity in rodent models of obese type 2 diabetes. PLOS ONE. 2015;10:e0116851.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Combination of the insulin sensitizer, pioglitazone, and the long-acting GLP-1 human analog, liraglutide, exerts potent synergistic glucose-lowering efficacy in severely diabetic ZDF rats. Diabetes Obes Metab. 2008;10:301-11.
- [CrossRef] [PubMed] [Google Scholar]
- Visceral fat and total body fat mass correlate differently with hormones in rat. Pathol Biol (Paris). 2008;56:283-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58:3977-83.
- [CrossRef] [PubMed] [Google Scholar]
- Antiadipogenic effects of a standardized quassinoids-enriched fraction and eurycomanone from eurycoma longifolia. Phytother Res. 2018;32:1332-45.
- [CrossRef] [PubMed] [Google Scholar]
- Erdheim-Chester disease: A rare multisystem histiocytic disorder associated with interstitial lung disease. Am J Med Sci. 2001;321:66-75.
- [CrossRef] [PubMed] [Google Scholar]
- Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta medica. 2001;67:628-33.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetics and tissue distribution of betulinic acid in CD-1 mice 1. Biopharm Drug Dispos. 1999;20:379-83.
- [CrossRef] [PubMed] [Google Scholar]
- Telmisartan prevents high-fat diet-induced hypertension and decreases perirenal fat in rats. J Biomed Res. 2012;26:219-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of Kurozu concentrated liquid on adipocyte size in rats. Lipids Health Dis. 2010;9:1-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antilipogenic effect of green tea extract in C57BL/6J-Lepob/ob mice. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2009;23:467.71.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-obesity effects of lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp Mol Med. 2011;43:205-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Palmiwon attenuates hepatic lipid accumulation and hyperlipidemia in a menopausal rat model. Menopause (New York, NY). 2015;22:872.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary intake of immature citrus tumida hort. ex tanaka peels suppressed body weight gain and fat accumulation in a mouse model of acute obesity. J Nutr Sci Vitaminol (Tokyo). 2019;65:19-23.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-Obesity and antidiabetic effects of nelumbinis semen powder in high-fat diet-induced obese C57BL/6 Mice. Nutrients. 2020;12:3576.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-γ in mice fed a high-fat diet. NRP. 2012;6:322.7.
- [CrossRef] [PubMed] [Google Scholar]
- IDDF2021-ABS-0197 Delayed intervention of agaropectin-derived oligosaccharides alleviate lipid accumulation by modulating intestinal flora homeostasis. BMJ Publishing Group; 2021.
- The hepatoprotective effect of the combination use of Fructus Schisandrae with statin–A preclinical evaluation. Journal of ethnopharmacology. 2016;178:104-14.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of electroacupuncture on serum ins and the fat content in male and female experimental obesity rats. Shanghai Journal of Acupuncture and Moxibustion 2017:94-7.
- [Google Scholar]
- Energy-restricted, high-protein diets more effectively impact cardiometabolic profile in overweight and obese women than lower-protein diets. Clinical Nutrition. 2017;36:371-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary fish oils limit adipose tissue hypertrophy in rats. Metabolism. 1990;39:217-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced meal frequency alleviates high-fat diet-induced lipid accumulation and inflammation in adipose tissue of pigs under the circumstance of fixed feed allowance. Eur J Nutr. 2020;59:595-608.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of aerobic exercises and dietary intervention on lipid metabolism in rats with metabolic syndrome and mechanism medicated by peroxisome proliferator-activated receptorα. Chinese Journal of Rehabilitation Theory and Practice 2017:662-6.
- [Google Scholar]
- Effects of preventive acupuncture at the fenglong acupoint on obesity prevention and PPAR-γ expression level in rats fed a high-fat diet. Chinese General Practice. 2019;22:707.
- [Google Scholar]
- Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A. 2017;114:8649-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel aspects of adipocyte biology: Activation of β3-adrenoceptors increases in vivo free fatty acid uptake andutilization in brown but not white fat depots in high-fat-fed rats. Am J Physiol Endocrinol Metab. 2016;311:E901.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genome-wide identification and characterization of long noncoding RNAs of brown to white adipose tissue transformation in goats. Cells. 2019;8:904.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in zucker diabetic fatty rats. PLOS One. 2014;9:e87936.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








