Translate this page into:
Pesticide Levels and Other Etiopathogenetic Factors in Patients with Chronic Kidney Disease of Unknown Cause in Central India – A Case–Control Study
Corresponding author: Mahendra Atlani, Department of Nephrology, All India Institute of Medical Sciences, Bhopal, India. E-mail: mkatlani9@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Atlani M, Kumar A, Srivastava A, Shrivastava A, Goel SK, Pakhare A, et al. Pesticide Levels and Other Etiopathogenetic Factors in Patients with Chronic Kidney Disease of Unknown Cause in Central India – A Case–Control Study. Indian J Nephrol. doi: 10.25259/IJN_95_2024
Abstract
Background
The etiology of chronic kidney disease of unknown cause (CKDu) remains unexplained, with environmental toxins, i.e., heavy metals and pesticides. being explored for their causal role. We measured pesticide levels in blood and urine in patients with CKDu in central India. We compared them with healthy and chronic kidney disease (CKD) controls.
Materials and Methods
This case–control study compared patients with CKDu (n = 55), CKD (n = 53) and healthy controls (n = 50). Levels of 6 organophosphates (OPs) and 16 organochlorines were measured by GC-MS. Drinking water sources and pesticide use, and hours spent in sunlight were also evaluated.
Results
CKDu and CKD subjects were age and sex matched. CKDu and CKD subjects had higher median chlorpyrifos (CP) 3.69 (2.36–5.65) and 3.79 (1.9–5.53) µg/L; pesticide use 19.6% and 12.5%; and heat spent hours 3.0 (2.0, 5.0) compared to healthy subjects 1.49 (0.97–2.20) µg/L; 0%; and 1.0 (1.0, 3.0) hours, respectively (p ≤ 0.001 for all). Surface water use was higher in CKDu (49%) compared to CKD (20.7%) and healthy subjects (20%) (p<0.01). The CP (ρ −0.0532, p<0.01), and ethion (ET) (ρ 0.221, p<0.01) had inverse correlation with GFR. Urine CP and ET were significantly higher in healthy controls. On multinomial regression, CP was independently associated with CKDu (OR, 95%CI) (3.5, 2.1–5.9) and CKD (3.7, 2.2–6.1). ET was also associated with CKDu (2.2, 1.2–3.9) and CKD (1.9, 1.1–3.4). Spending 4 hours or more in sunlight was associated with CKDu (6.1, 1.7–22.3) and CKD (6.0,1.7–21.3) (P<0.01 for all) in reference to healthy subjects. Surface water was associated with CKDu (4.0, 1.3–12.7) (p<.01).
Conclusion
Environmental factors such as spending 4 hours or more in sunlight and higher levels of OP pesticides, namely, CP and ET, are associated with both CKDu and CKD. As higher levels of pesticides were seen in both groups of CKDu and CKD, the association of pesticides with CKDu could not be established. The higher levels could be due to low eGFR. Surface water use is independently associated with CKDu; however, larger studies are required to establish the causation.
Keywords
CKDu
Surface water
Pesticides in CKDu
Heat stress in CKDu
Environmental toxins in CKDu
Introduction
Chronic kidney disease of unknown cause (CKDu) is a diagnosis of exclusion that refers to CKD without an identifiable risk factor.1 It has been reported from rural agricultural communities belonging to lower socioeconomic strata in different parts of the world, i.e., Central America, Sri Lanka, and India. In India, prevalence has been reported to be between 13 and 40% from various parts.2 The renal histology shows dominance of interstitial fibrosis and tubular atrophy, with variable interstitial inflammation, ischemic changes, and glomerular obsolescence.3
As the disease (CKDu) is more common in people associated with occupations like agriculture, environmental factors are considered responsible. The available evidence regarding the association of environmental factors and CKDu remains inconclusive at present; both positive association and lack of association have been reported with CKDu and various environmental factors, i.e., heavy metals, heat stress pesticides, etc. Other than these environmental factors, herbal medicines, infections, i.e., leptospirosis, and even genetic factors have also been found to be associated with CKDu.4
Studies have reported drinking groundwater with higher levels of fluoride,5 silica, and strontium6 or higher hardness of water7 to be associated with CKDu. Higher levels of cadmium8 and lead9 have been reported in biological samples of CKDu patients. Whereas, an another study did not find association of Cd or As with CKDu, on analysis of urine, hair or nails and renal tissue.10
Regarding pesticides, the emergence of CKDu in the 1990s coincides with increased pesticide use. Various studies reported an association between CKDu and pesticides based only on questionnaires in the form of yes/no;11,12 only a few studies measured pesticide levels in study subjects. An Indian study13 measured blood organochlorines (OC) in patients with CKDU and reported an association of higher blood levels of some OCs with CKDu. A study from Sri Lanka reported higher well water glyphosate (an herbicide) and association with CKDu in the population drinking water from such abandoned wells.14 On the contrary, a 2018 systematic review and meta-analysis of epidemiological studies of the Mesoamerican region did not find an association between CKDu and pesticides.15 An Indian study also reported undetectable pesticide residues in cases (CKDu, CKD) as well as controls.16
There is new evidence in patients with Mesoamerican nephropathy (MeN) that long hours of heat exposure result in pre-renal AKI, hyperuricemia, and its conversion to glucosamine, causing tubulointerstitial injury.17 Whereas some researchers from the same geographical areas of MeN, by analyzing trends and the mortality pattern among women, children, and adolescents,18 reported that the heat stress–dehydration hypothesis could not fully explain the diseases. The evidence in favor of heat stress for endemic nephropathies from other than MeN is also not available.
Some studies have also found the role of genetic factors. A study reported the association of single nucleotide polymorphisms in KCNA10 and SLC13A3 genes with CKDu,4 and another study reported the role of GSTM1/GSTT1 polymorphism in CKDu.19
Overall, available evidence regarding the association of environmental factors and CKDu remains inconclusive. We planned to measure both OC and organophosphate (OP) pesticides in patients with CKDu in central India and compare it with healthy as well as CKD controls to find if there is any differential association of higher serum levels of pesticides with CKDu in this geographical area compared to CKD and healthy controls. We also planned to observe the association of source of drinking water, pesticide use, and hours spent in sunlight in the daytime (heat stress) with CKDu.
Materials and Methods
The study was conducted in central India in a case–control design between June 2020 and 2022. Participants were enrolled between June 2020 and December 2021. The data collection was done simultaneously. The sample analysis was carried out between January and June 2022. The study was performed according to the guidelines of the Declaration of Helsinki. The study included adults aged 18–70 years with CKDu and two groups of the control population, one with CKD and another group of healthy controls. The internal ethics committee cleared the study, and a permission letter was issued.
The CKDu and CKD cases were inducted among the patients visiting the nephrology outpatient department based on predefined criteria. At the same time, healthy controls were inducted from outpatient departments. Written informed consent was obtained from all the participants.
The case definition of CKDu was based on criteria proposed previously to diagnose CKDu.20 The inclusion criteria included – eGFR (estimated glomerular filtration rate) < 60 mL/min/1.73m2 (CKD-EPI)21 and albumin-to-creatinine ratio (ACR) > 30 mg/g for more than 3 months with (1) urine protein creatinine ratio (uPCR) less than 2 g/g; (2) no history of pyelonephritis, renal calculi, polycystic kidneys, or obstruction on renal ultrasound, (3) not on treatment for diabetes and HbA1c less than 6.5%, (4) blood pressure less than 140/90 if CKD stages 1 and 2 and less than 160/100 if CKD stages 3,4, and 5 and on a single drug for blood pressure control, and (5) urine RBC < 5/HPF.
Inclusion criteria for healthy controls included – eGFR >90 mL/min/1.73m2; ACR < 30 mg/g and lack of anatomical renal disease; obstruction or stone on renal ultrasound; no history of diabetes; HbA1C less than 6.5; and BP less than 140/90.
The case definition and staging of CKD was based on criteria recommended by the Kidney Disease Quality Outcome Initiative (K/DOQI).22
The study compared CKDu cases with age and sex-matched CKD and healthy controls in a 1:1:1 ratio. Exposures included serum and urine levels of various OC and OP pesticides. Potential confounders’ data included source of drinking water, pesticide use, hours spent in sunlight in the daytime (heat stress), and glomerular filtration rate (GFR).
A predefined pro forma was utilized to collect case details. We collected details on the number of years in contact with pesticides, type of contact – mixing/spraying or both, type of pesticide used (OP/OC), method of disposal, and any hospitalization after using pesticide.
For heat stress evaluation, a pro forma with predefined questions was used. Heat stress was defined as “hours spent in day time in sunlight between 10 AM and 5 PM round the year.” Questions evaluated included numbers of hours spent during day time in the fields or other occupations between 10AM and 5PM, breaks taken during work hours (yes/no), shades used (yes/no), rehydration used (yes/no), average temperature of climate in summers in area of patient residence and hospitalization due to heat effect.
Questions evaluated source of drinking water as to whether bore well, hand pump, or well water (ground water) is used, or whether water supplied by the municipal body or by tankers from rivers/lakes (surface water) is used for drinking and was appropriately categorized as ground or surface water for analysis. Data on the type of profession, area of residence, and years in area of residence were also noted.
Biases were kept at a minimum by adhering to the predefined case–control definitions, and study exposures were objectively assessed. The staff collecting the data was masked to a category to which the study subjects belonged, either cases or control.
Specimen collection and analysis
Information on demography and exposure variables was registered in a predesigned pro forma. For the analysis of pesticides, venous blood 10 mL per participant was centrifuged at 900 × g. The separated serum was stored at −40°C until chemical analysis. Ten milliliters of first-morning urine was collected in 50 mL polypropylene tubes. Urine was stored at −40°C in aliquots until analysis. Levels of pesticides were measured in subjects’ serum and urine at the Centre for Advance Research, KGMU, Lucknow (India). A gas chromatography (GC) (Thermo scientific trace 1300) hyphenated technique with a highly precise TRIPLUS 100 auto-sampler was used to separate metabolites. Pesticide detection was performed using mass spectrometry (Thermo scientific TSQ 8000) with electron ionization mode. All samples were analyzed in the selected reaction monitoring mode. Extraction and clean-up of pesticide residues and method validation parameters are included in the Supplementary File 1. Chemical and pesticide standards were of high-quality analytical grade from MERCK, FINAR, Sigma-Aldrich, Bengaluru, India, Agilent Technologies-USA, and RESTEK [Supplementary File 1]. The urine pesticide levels were adjusted for urine dilution by estimating pesticides per gram of creatinine in urine.
Sample size
Assuming a difference of moderate effect size (0.25) between three groups (CKDu cases, CKD controls, and healthy controls) with a confidence level of 95% and power of 80%, the calculated sample size was 159. The final sample size estimated, including a 10% nonresponse rate, was 180 (60 per group).
Statistical methods
Statistical analyses were performed with R version 4.2 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS 26. Data are presented as percentages for categorical variables or as median (Q1–Q3) for continuous variables. The data distribution in groups was evaluated with Shapiro–Wilk, kurtosis, and skewness; skewed data for three groups was compared with the Kruskal–Wallis test. The Dunn test performed subgroup analysis in three groups with pairwise comparison. Parameters with homogeneous distribution were compared with the chi-square test.
Detection rates for serum pesticides were calculated. The Spearman correlation coefficient was calculated to find a correlation with GFR. The list-wise deletion was used to handle missing data. Age and gender matching was done to control confounding.
We performed multinominal logistic regression analysis to identify independent factors associated with CKDu. Factors having a p-value less than 0.01 on univariate analysis were included for multinominal regression analysis. We have considered a p-value less than 0.05 as statistically significant for all analyses.
Results
A total of 568 patients were screened for inclusion. Out of these, 61 CKDu and 56 CKD cases were found to be eligible to enroll in the study. We have approached 112 healthy persons accompanying patients attending other OPDs. Out of these, 60 were eligible, and 53 provided their consent. In the final analysis, subjects with missing data were excluded, and 55 cases in CKDu, 53 in CKD, and 50 in the healthy control group were included [Figure S1].
Demography and Lab parameters
The CKDu and CKD subjects were similar in demographics for age and sex [Table 1]. The patients in the CKD group were as follows: 05 diabetic kidney disease, 04 CKD due to secondary glomerular disease (3-lupus nephritis, 1-FSGS), 12 hypertension-associated renal disease, 01 ADPKD, 30 chronic glomerulonephritis, and 04 chronic pyelonephritis. There were 14 cases with confirmed etiologies in the CKD group. In the CKDu group, 73.5% (n = 39/53) patients, in the CKD group 74.5% (n = 41/55) patients, and among healthy controls 78% (n = 39/50) were from Bhopal and its vicinity of 150 km which included nearby districts–Raisen Sehore, Vidisha, Hoshangabad, and Sagar.
| Population Characteristics | CKDu, N = 55 | CKD, N = 53 | Healthy, N = 50 | p-value | CKD vs. CKDu* | CKDu vs. Healthy* | CKD vs. Healthy* |
|---|---|---|---|---|---|---|---|
| Age (Years)1 | 42.0 (34.5, 50) | 43 (31, 52) | 31.0 (27.0, 41) | 0.00# | 0.70 | 0.00 | 0.00 |
| Gender (male)2 | 30 (54.5) | 30 (56.6) | 33 (66) | 0.48@ | 0.5 | 0.21 | 0.37 |
| SBP (mmHg)1 | 138 (124.0, 144.0) | 142.0 (138.0, 154.0) | 125.0 (116.8, 132.0) | 0.00# | 0.00 | 0.00 | 0.00 |
| DBP (mmHg)1 | 89.0 (80.0, 94.0) | 89.5 (83.0, 97.8) | 86.0 (73.2, 89.0) | 0.00# | 0.11 | 0.01 | 0.00 |
| Alcohol use (yes)2 | 8 (14.5) | 4 (7.5) | 3 (6) | 0.2$ | 0.15 | 0.17 | 0.94 |
| Smoking (yes)2 | 2 (3.6) | 5 (9.4) | 4 (8) | 0.4$ | 0.19 | 0.30 | 0.79 |
| Pesticide use (yes)2 | 12 (21.8) | 7 (13.2) | 0 (0) | 0.00$ | 0.2 | 0.00 | 0.00 |
| Source of drinking water (surface water)2 | 27 (49) | 11 (20.7) | 10 (20) | 0.00$ | 0.00 | 0.00 | 0.84 |
| Duration of stay (years) mean+/−SD | 27+/−16.7 | 28+/−16.7 | 14.98+/−10.6 | 0.00## | |||
| Heat exposure (hours/day)1 | 3.0 (2.0, 5.0) | 3.0 (2.0, 5.0) | 1.0 (1.0, 3.0) | 0.00# | 0.69 | 0.00 | 0.00 |
| Occupation (farmer and labor work)2 | 18 (34) | 23 (42) | 7 (14) | 0.00$ | |||
| ACR1st (mcg/mg)1 | 411.5 (79.2, 677.5) | 1,301.9 (602.0, 3,495.0) | 13.9 (6.6,17.8) | 0.00# | 0.00 | 0.00 | 0.00 |
| PCR (g/g)1 | 1.29 (0.4, 1.8) | 3.2 (2.3, 6) | 0.04 (0.02,0.07) | 0.00# | 0.00 | 0.00 | 0.00 |
| HbA1C (pct)1 | 5.4 (5.1, 5.8) | 5.4 (5.1, 5.7) | 5.8 (5.2, 6.0) | 0.12# | 0.9 | 0.12 | 0.4 |
| eGFR (mL/min/1.73m2)1 | 13.0 (7.0, 33.0) | 8.0 (4.0, 17.0) | 109 (95, 122) | 0.000# | 0.703 | 0.00 | 0.00 |
1Median (IQR) and 2percentage; @Pearson’s Chi: squared test; $Fisher’s exact test, #Kruskal: Wallis rank sum test; *Dunn’s test; ## One-way ANOVA. CKD: chronic kidney disease, CKDu: chronic kidney disease of unknown cause, SBP: systolic blood pressure, DBP: diastolic blood pressure, ACR1st: albumin creatinine ratio on first visit, Sr: serum, eGFR: estimated glomerular filtration rate, PCR: protein creatinine ratio, Pct: percentage, HbA1C: Glycocylated hemoglobin.
A significant difference was found concerning the source of drinking water (ground or surface water) [Table S1 and Figure S2]. The mean duration of residence was not different for CKDu and CKD but was different with respect to healthy controls [Table 1].
A higher number of CKDu subjects reported pesticide use. The numbers of residents who were involved in pesticide use were n = 12/53 in CKDu and n = 7/55 in CKD, Table 1; subjects involved in pesticide use either performed mixing or spraying or both for a median (with 25-75 quartiles) of 18 (11.25-25) years in CKDu and 20 (12-25) years in CKD subjects (p>.05). There was no pesticide user in healthy controls. However, exact days per year during which residents were involved in mixing or spraying or both were not reported by the pesticide users.
Subjects with CKDu and CKD had significantly higher heat exposure. Based on annual average temperature (28.710C) and relative humidity (58%) of Madhya Pradesh, a heat stress index of 29.46 is found. Significantly more subjects with CKD and CKDu were associated with occupations like farming and manual labor [Table 1]. Blood pressures were significantly higher in CKD subjects than in CKDu and healthy subjects. Both ACR and PCR were also significantly different between CKD and CKDu. The eGFR was not significantly different between the CKD and CKDu subjects. Only subjects with nonmissing data were included for the final analysis.
Pesticide analysis results
Twenty-two types of pesticides were measured in the serum, in concentration of parts per billion (ppb) or micrograms per liter. Six of these were organophosphorus (OP) and sixteen belonged to the OC category. Among the OPs, phorate, phorate sulfone, ethion (ET), malathion, CP, and CP-methyl were measured. In the OC category, aldrin, alfa, and beta-endosulfan; four isomers of hexachlorocyclohexane (HCH)-alfa, beta, delta, and gamma HCH (lindane); Cis and trans chlordane; heptachlor and heptachlor epoxide; P’P and O’P-DDE; and endrin and endrin-aldehyde were measured.
Among the OPs, CP, ET, and phorate were detectable in serum [Table 2], whereas phorate sulfone, malathion, and CP-methyl were undetectable [Table S2]. Among the OCs, only aldrin, alfa, beta-endosulfan, delta and gamma HCH (lindane), Cis, and trans chlordane were measurable [Table 2]. In contrast, alfa and beta HCH; heptachlor and heptachlor epoxide; P’P and O’P-DDE; endrin and endrin-aldehyde were unmeasurable [Table S2].
| Pesticide | CKDu N = 53 | CKD N = 55 | Healthy N = 50 | P-value 1&2 | CKDu vs Healthy | CKD vs Healthy | CKDu vs CKD |
|---|---|---|---|---|---|---|---|
| Chlorpyrifos* | 3.69 (2.36–5.65) | 3.79 (1.9–5.53) | 1.49 (0.97–2.20) | 0.000 | 0.000 | 0.000 | 0.402 |
| Samples with levels detected (%) | 98 | 94.5 | 84 | 0.02 | 0.01 | 0.08 | 0.29 |
| Correlation with GFR (rs) | −0.532** | ||||||
| Urine Chlorpyrifos (op)* | 1.292 (0.00–2.191) | 0.946 (0.00–2.10) | 2.27 (0.971–3.59) | 0.001 | 0.017 | 0.000 | 0.136 |
| Ethion* | 0.00 (0.00–2.26) | 0.00 (0.00–1.68) | 0.00 (0.00–0.00) | 0.000 | 0.000 | 0.005 | 0.227 |
| Samples with levels detected (%) | 43 | 31 | 12 | 0.003 | 0.00 | 0.02 | 0.18 |
| Correlation with GFR (rs) | −0.221** | ||||||
| Urine Ethion (op)* | 0.00 (0.00–0.449) | 0.00 (0.00–0.182) | 0.00 (0.792–2.69) | 0.000 | 0.000 | 0.000 | 0.129 |
| Serum phorate* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.45 | |||
| Samples with levels detected (%) | 5.6 | 0 | 6 | 0.16 | |||
| Correlation with GFR (rs) | 0.123 | ||||||
| Urine phorate (OP)* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00-0.00) | 0.028 | 0.011 | 0.049 | 0.589 |
| Aldrin* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.10 | 0.01 | 0.3 | 0.10 |
| Samples with levels detected (%) | 13 | 7.3 | 0 | 0.02 | 0.00 | 0.05 | 0.35 |
| Correlation with GFR (rs) | −0.097 | ||||||
| Urine Aldrin* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.688 | |||
| Alpha-Endosulfan* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.2 | 0.3 | 0.08 | 0.5 |
| Samples with levels detected (%) | 5.6 | 5.5 | 0 | 0.249 | |||
| Correlation with GFR (rs) | −0.134 | ||||||
| Urine Alpha-endosulfan* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | |||
| Lindane (y-HCH)* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.230 | 0.2 | 0.2 | 0.9 |
| Samples with levels detected (%) | 13 | 11 | 4 | 0.251 | |||
| Correlation with GFR (rs) | −0.102 | ||||||
| Urine Lindane* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | |||
| Delta HCH* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00 | 0.173 | 0.9 | 0.1 | 0.09 |
| Samples with levels detected (%) | 0 | 3.6 | 0 | 0.330 | |||
| Correlation with GFR (rs) | −0.054 | ||||||
| Urine delta HCH* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | |||
| Cis-chlordane* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.301 | 0.4 | 0.2 | 0.7 |
| Samples with levels detected (%) | 5.6 | 3.6 | 10 | 0.387 | |||
| Correlation with GFR (rs) | 0.142 | ||||||
| Urine Cis-chlordane* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | |||
| Trans-chlordane* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.283 | 0.3 | 0.3 | 0.6 |
| Samples with levels detected (%) | 5.6 | 3.6 | 10 | 0.392 | |||
| Correlation with GFR (rs) | 0.178** | ||||||
| Urine trans-chlordane* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | |||
| Beta-endosulfan* | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.926 | 0.7 | 0.7 | 0.4 |
| Samples with levels detected (%) | 7.4 | 5.5 | 10 | 0.651 | |||
| Correlation with GFR (rs) | 0.039 | ||||||
| Urine Beta –endosulfan* | 0.00 (0.00-0.000) | 0.00 (0.00-0.000) | 0.00 (0.00-0.000) | 1.000 |
Serum CP and ET had significantly higher detection rates and levels in subjects with renal dysfunction (CKD and CKDu) than in healthy subjects [Figure 1 and Figure S3]. There was a significant negative correlation of serum CP and ET with GFR [Table 2]. Significantly higher urinary concentration of these pesticides was found in healthy controls as compared to subjects with CKDu and CKD [Table 2].
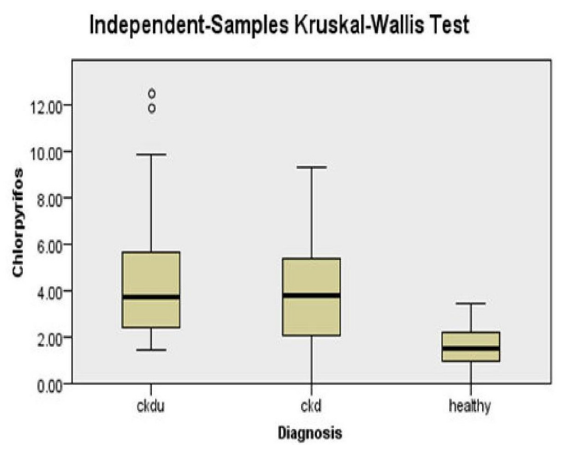
- Serum chlorpyrifos between CKDu, CKD, and healthy subjects.CKD: Chronic kidney disease, CKDu - CKD of unknown cause.
The levels of CP and ET were not significantly different between CKDu and CKD subjects. However, these were significantly different with respect to healthy subjects. Among the OC, serum aldrin levels and detection rates were significantly higher in subjects with renal dysfunction (CKD and CKDu) than healthy subjects. There was a significant negative correlation of serum aldrin with GFR. There was no significant difference in serum levels of aldrin in CKDu and CKD groups; however, there was a significant difference between CKDu and healthy subjects.
Alfa and beta-endosulfan; delta and gamma HCH (lindane); Cis; and trans-chlordane levels were not significantly different between CKDu, CKD, and healthy subjects.
Regression analysis
Regression analysis was done to see the independent effect estimates of associated factors with CKDu. The unadjusted model [Table S3] had a good fit and showed that serum CP was associated with CKDu (OR 2.7 95% CI 1.8–4.0, p<0.001) and CKD (OR 2.8 95% CI 1.9–4.3, p<0.001) and ET was also associated with CKDu (OR 2.1 95%CI 1.2–3.5, p<0.006) and CKD (OR 1.8 95%CI 1.1–3.1, p = 0.02), respectively, with reference to healthy subjects. After adjusting for confounding factors, i.e., age, surface water usage for drinking water, and spending 4 hours in heat, serum CP and ET remained associated with CKDu and CKD [Tables 3]. Heat spent hours were recategorized as less than 4 hours or equal to and more than 4 hours in sunlight. As the level of GFR was an integral part of diagnostic categories, the results were not adjusted for GFR.
| Multinominal logistic regression between CKDu and healthy groups | ||
|---|---|---|
| Parameters | OR CI (95%) | P-value |
| Age | 1.1 (1.0–1.1) | 0.000 |
| Spending 4 or more hours in heat | 6.1 (1.7–22.3) | 0.005 |
| Chlorpyrifos | 3.5 (2.1–5.9) | 0.000 |
| Ethion | 2.2 (1.2–3.9) | 0.004 |
| Drinking water source (surface water) | 4.0 (1.3–12.7) | 0.002 |
| Multinominal logistic regression between CKD and healthy groups | ||
| Age | 1.0 (1.0–1.1) | 0.001 |
| Spending 4 or more hours in heat | 6.0 (1.7–21.3) | 0.005 |
| Chlorpyrifos | 3.7 (2.2–6.1) | 0.000 |
| Ethion | 1.9 (1.1–3.4) | 0.017 |
| Drinking water source (surface water) | 2.2 (0.56–8.7) | 0.253 |
OR: Odds ratio, CI: Confidence interval, Final Model Fitting sig.−0.000 and Goodness-of-fit-Pearson −0.930, CKD: Chronic kidney disease, CKDu: Chronic kidney diseaseof unknown cause.
Discussion
In the current study, serum CP and ET levels were significantly higher in patients with renal dysfunction, i.e., CKDu and CKD, compared to healthy subjects. These pesticides had a significant negative correlation with GFR; the unadjusted and adjusted (for age, surface water use, and heat spent hours) levels of these OP pesticides (CP and ET) remained independently associated with patients with CKDu and (CKD) compared to healthy subjects. To our knowledge, this is the first study from central India in which OCs and OPs were measured to find an association with CKDu and multiple controls, including CKD patients and healthy subjects.
A clustering of cases of CKDu was not found different from CKD and healthy controls, hence, an endemicity cannot be labeled in this study. The study period was affected by covid-19 pandemic, which could have affected the movement of patients from areas usually well within the reach of the study center.
The higher OP levels compared to OCs in the current study may be related to central India’s current pesticide use pattern. According to a survey among farmers of the Sagar district, located in Madhya Pradesh in central India, CP is the pesticide most commonly used by these farmers.23 Seventy-six percent of the evaluated samples of vegetables from the farmers in this area showed residues of CP. Previous studies have reported the detection of OC residues instead of OPs, which is in contrast to the observed results in the present study. A study from Punjab (India), the highest pesticide user in India, reported the absence of OP residues in study subjects.24 In this study, we found that CP and ET are associated with patients with reduced renal functions, i.e., CKDu and CKD. Both these pesticides had an inverse correlation with GFR. This indicates that the raised levels are because of poor excretion, which may or may not be associated with disease pathogenesis. Association with both the diseases (CKDu and CKD) suggests that the presumed explanation may be correct. This is also supported by the significantly higher levels of these pesticides in urine of healthy controls with normal renal function.
Various OPs have been found to stimulate oxidative stress, and each OP has a unique toxicity profile.25 In the case of CP, evidence exists that it is associated with oxidative stress and apoptosis of renal cells.26 As there is evidence from the studies that pesticide exposure is associated with oxidative stress and inflammatory markers, it may be considered that accumulated pesticides may be associated with the maintenance and progression of kidney injury.27 The association of pesticides with CKDu and CKD remains statistically significant even after adjustment for various confounders, i.e., heat spent hours; it may be considered that the second explanation that accumulated pesticides may be associated with the maintenance and progression of kidney injury may also hold true about the association of pesticides in patients with reduced renal functions.
In accordance with the current study, another study from north India, which studied OC pesticides in patients with CKDu, CKD, and healthy controls, also reported raised OCs in CKD and CKDu patients compared to healthy controls; a negative correlation with GFR was also reported.13 They reported higher beta-endosulfan and p, p′-DDE in CKDu patients compared to CKD patients.
Sufficient evidence to establish causality for agrochemical usage as a putative exposure for CKDu is not available. Differences in exposure measurement between studies may have contributed to some case–control and prevalence studies showing a strong association,14,28,29 while others have shown a weak or no association.30,31 A Sri Lankan study found lower neonicotinoid insecticide in farmers with CKDu compared to farmers without CKDu from the noncentral region of the country, but when results were adjusted for creatinine excreted, the difference disappeared. The study found no difference in neonicotinoid insecticide levels of farmers with and without CKDu.32
As mentioned above, a 2018 systematic review and meta-analysis of epidemiological studies of the Mesoamerican region did not find any association between CKDu and pesticides,15 whereas a clear link between agrochemical usage and CKD has been demonstrated in other settings. A study found lower levels of blood acetyl choline esterase (AChE) in CKD farmers than controls, which can be due to exposure to pesticides that lower AChE. AChE levels recover within 3 months, but CKD takes years to progress, so the association does not appear to be causative.33
A US study utilizing data from NHANES surveys found malathion to be associated with CKD without diabetes and hypertension. It was a subgroup analysis and the diagnostic criteria of CKDu needed to be better defined. The CKDu group may have included patients with CKD due to other causes of CKD, i.e., pyelonephritis, stones, etc.34 Dietary evaluation was not a part of the study; however, drinking water sources, either groundwater or surface water use, were evaluated [Table-S1, Figure-S2]. Surface water use was independently associated with CKDu. However, this outcome should be seen with caution as though the duration of stay in the informed geographical location is quite long, the sources of drinking water may have changed over the years. Surface water source pollution with pesticides and its accompanying heavy metals (arsenic) is common,35 and water consumption from these sources may be a source of exposure in these subjects. In another analysis of our study subjects for heavy metals, we reported significantly higher blood arsenic levels in CKDu patients than in CKD and healthy controls.36 Heat stress with a resulting muscle injury, reduced renal blood flow, and fructose metabolism may induce kidney inflammation, and its impaired resolution by daily repeating pro-inflammatory triggers can cause CKDu.17 Heat stress was independently associated with CKDu and CKD in our study. To the best of our knowledge, this is the first study wherein we have compared CKD and CKDu subjects for heat stress. As mentioned above, calculated heat stress index for Madhya Pradesh is 29.46, based on data from https://www.indianclimate.com. Our study shows that spending 4 hours or more in an environment with heat stress index of 29.46 is associated with CKDu and CKD.
Our study has some limitations - the study subjects were age-matched in the CKD and CKDu groups, but the healthy subjects were younger. As the study was conducted in the geographical area of central India, the generalizability of results should be used cautiously. The study’s sample size is small. Potential recall bias cannot be ruled out completely. The researcher bias was minimized by masking the data collector for diagnostic categories of study subjects.
Conclusion
The study shows various environmental factors, such as spending 4 hours or more in the heat, and higher levels of OP pesticides, CP, and ET are associated with CKDu and CKD. Surface water use is independently associated with CKDu. As higher levels of pesticides were seen in both groups of CKDu and CKD, the association of pesticides with CKDu could not be established. The association of pesticides with patients with reduced renal functions may be due to reverse causation or poor excretion with decreasing renal functions. Heat stress is also associated with both CKDu and CKD. Further studies with improved study design involving a larger population are required to understand better the etiopathogenesis of CKDu and the role of OPs and surface water use in this context.
Acknowledgements
We thank the Indian Council of Medical Research (ICMR), New Delhi, India, for funding the study, with sanction no. :5/4/7-14/2019-NCD-II.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of interest
There are no conflicts of interest.
References
- The international society of nephrology’s international consortium of collaborators on chronic kidney disease of unknown etiology: Report of the working group on approaches to population-level detection strategies and recommendations for a minimum dataset. Kidney Int. 2019;95:4-10.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease of unknown etiology: Hotspots in India and other Asian countries. Semin Nephrol. 2019;39:272-27.
- [CrossRef] [PubMed] [Google Scholar]
- Pathology of uddanam endemic nephropathy. Indian J Nephrol. 2020;30:253-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of single nucleotide polymorphisms in KCNA10 and SLC13A3 genes with the susceptibility to chronic kidney disease of unknown etiology in central Indian patients. Biochem Genet. 2023;61:1548-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dose-dependent Na and Ca in fluoride-rich drinking water–another major cause of chronic renal failure in tropical arid regions. Sci Total Environ. 2011;409:671-5.
- [CrossRef] [PubMed] [Google Scholar]
- Role of drinking water with high silica and strontium in chronic kidney disease: An exploratory community based study in an Indian Village. Indian J Community Health. 2015;27:95-102.
- [Google Scholar]
- Drinking water quality and chronic kidney disease of unknown etiology (CKDu): Synergic effects of fluoride, cadmium and hardness of water. Environ Geochem Health. 2016 Feb;38:157-68.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evidence of selected nephrotoxic elements in Sri Lankan human autopsy bone samples of patients with CKDu and controls. BMC Nephrol. 2020;21:384.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Systematic evaluation of exposure to trace elements and minerals in patients with chronic kidney disease of uncertain etiology (CKDu)in Sri Lanka. J Trace Elem Med Biol. 2019;54:206-13.
- [CrossRef] [PubMed] [Google Scholar]
- Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the agricultural health study. Occup Environ Med. 2016;73:3-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Environmental factors incriminated in the development of end stage renal disease in el-Minia governorate, Upper Egypt. Int J Nephrol Urol. 2010;2:431-7.
- [Google Scholar]
- Organochlorine pesticide level in patients with chronic kidney disease of unknown etiology and its association with renal function. Environ Health Prev Med. 2017;26;22:49.
- [CrossRef] [PubMed] [Google Scholar]
- Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease in Padavi-Sripura, Sri Lanka. Environ Health. 2015;18;14:6.
- [CrossRef] [PubMed] [Google Scholar]
- What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin Kidney J. 2018;11:496-506.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Heavy metals and pesticides in chronic kidney disease–results from a matched case-control study from a rural population in Shivamogga District in South India. Indian J Nephrol. 2019;29:402-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients. 2020;12:1639.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease mortality trends in selected central America: Clues to an epidemic of chronic interstitial nephritis of agricultural communities. J Epidemiol Community Health. 1978;72:280.
- [Google Scholar]
- Increased level of organochlorine pesticides in chronic kidney disease patients of unknown etiology: Role of GSTM1/GSTT1 polymorphism. Chemosphere. 2014;96:174-9.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease of unknown etiology: Case definition for India - A perspective. Indian J Nephrol. 2020;30:236-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CKD-EPI (chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (KDIGO) Kidney Int. 2005;67:2089-100.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of most commonly used pesticides in green leafy vegetables from sagar, india using direct injection hybrid micellar liquid chromatography. Advances in Sample Preparation. 2022;2:100015.
- [CrossRef] [Google Scholar]
- Evaluation of pesticide residues in human blood samples from Punjab (India) Vet World. 2015;8:66-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular mechanisms of acute organophosphate nephrotoxicity. Int J Mol Sci. 2022;23:8855.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chlorpyrifos-induced oxidative stress and histological changes in retinas and kidney in rats: Protective role of ascorbic acid and alpha tocopherol. Pesticide biochemistry and physiology. 2010;98:33-8.
- [CrossRef] [Google Scholar]
- Study on organochlorine pesticide levels in chronic kidney disease patients: Association with estimated glomerular filtration rate and oxidative stress. J Biochem Mol Toxicol. 2012;26:241-7.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease of unknown aetiology in Sri Lanka: is cadmium a likely cause? BMC Nephrol. 2011;12:32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dehydration and malaria augment the risk of developing chronic kidney disease in Sri Lanka. Indian J Nephrol. 2015;25:146-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011;80:1212.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors for impaired kidney function in the district of Anuradhapura, Sri Lanka: A cross-sectional population-representative survey in those at risk of chronic kidney disease of unknown aetiology. BMC Public Health. 2019;19:763.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neonicotinoid concentrations in urine from chronic kidney disease patients in the north central region of Sri Lanka. J Occup Health. 2016;58:128-33.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to acetylcholinesterase-inhibiting pesticides and chronic renal failure. Ceylon Med J. 2006;51:42.
- [CrossRef] [PubMed] [Google Scholar]
- Association of pesticides and kidney function among adults in the US population 2001-2010. Int J Environ Res Public Health. 2021;18:10249.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Water pollution with special reference to pesticide contamination in India. J Water Resource and Protection. 2010;2:432-48.
- [CrossRef] [Google Scholar]
- Heavy metal association with chronic kidney disease of unknown cause in central India-results from a case control study. BMC Nephrol. 2024;25:120.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








