Translate this page into:
Phaeohyphomycosis in Renal Transplant Recipients: A Case Series
Address for correspondence: Dr. Pallavi Prasad, 37 & 38, Sri Lakshmi Nagar, 10th Cross Street, Valsarwakkam, Chennai - 600 087, Tamil Nadu, India. E-mail: pallaviprasad1986@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Phaeohyphomycosis is a rare fungal infection in renal transplant recipients. We describe here five cases of phaeohyphomycosis in renal transplant recipients, two with deep-seated unusual sites of infection. All patients received antifungals, and surgical excision was done where feasible. Outcomes included complete resolution of infection in three, partial resolution in one, and mortality in one case.
Keywords
Dematiaceous fungi
fungal infection
phaeohyphomycosis
renal transplantation
Introduction
Dematiaceous fungi are rare, pigmented fungi in renal transplant recipients and may cause phaeohyphomycosis, mycetoma, or chromoblastomycosis.[1] The most common presentation is with cutaneous lesions, but deep-seated organ involvement can also occur.[2] We describe here five cases of phaeohyphomycosis in renal transplant recipients which occurred over a period of 10 years at our center.
Case Series
Case 1
A 50-year-old male case of live related renal transplant and treated chronic hepatitis C presented 2 years after his transplant with an indolent swelling on the posterior aspect of the left thigh for one month [Figure 1] and a small swelling on the left foot. There was no h/o fever, local trauma, insect bite or prior skin rash at the site. The swelling was firm to touch and non-fluctuant.

- A phaeohyphomycotic cyst on the dorsal surface of thigh
Investigations revealed a slightly increased TLC (11,700 cells/mm3, 56% neutrophils) and a normal graft function (serum creatinine – 1.0 mg/dl). An incisional biopsy of the swelling was done, which showed acute on chronic inflammation with granuloma formation with multinucleated giant cells. Scattered fungal hyphae with constrictions at site of branching and brown melanin pigmentation were also seen suggestive of phaeohyphomycosis. Fungal stains were positive although culture grew coagulase negative staphylococcus. AFB stain was negative.
The patient was advised surgical excision of the lesion but was not willing for the same. Oral itraconazole 100 mg twice daily was started and tacrolimus dose was decreased. At 1 month of itraconazole therapy, there was partial regression of the lesion. The patient was lost to follow up for 1 year after which he presented to the transplant clinic with further regression of the size of lesion in the popliteal fossa and the foot lesion had been excised elsewhere. He was not willing for surgical excision of the remnant lesion or further antifungal therapy.
Case 2
A 55-year-old male presented 3 months after kidney transplantation with a history of pain and swelling in the pulp space of the second right phalanx and dorsum of the right foot. He also had a mildly purulent discharge from the swelling on the right hand. The swellings were fluctuant and tender. He had a history of minor skin trauma while working in rice fields. Investigations revealed a TLC of 15800 cells/mm3 (94% neutrophils) and a rise in serum creatinine from 1.5 to 2.5 mg/dL. He was started on antibiotics. Incision and drainage of both swellings was done. Pus culture from the swelling grew Acinetobacter and blood cultures were sterile. AFB stain and nucleic acid amplification test for M. tuberculosis were negative. The patient failed to improve with antibiotics and hence excision biopsy of foot lesion was done. It showed fibrocollagenous tissue with necrotic debris. Short fungal hyphae with constriction bands at branching site and bulbous ends were seen with few budding spores. The patient was started on voriconazole and antibacterials were also continued. Both tacrolimus and mycophenolate were discontinued. However, the patient worsened and developed pancytopenia, worsening graft function and shock. Antifungal was changed to caspofungin, but the patient failed to improve and succumbed to his illness.
Case 3
A 35-year-old kidney transplant recipient with a history of chronic allograft injury presented 2 years post transplantation with a history of swelling and pain on the sole of the left foot for 2 weeks. No history of fever, weight loss, trauma or obvious skin breach was observed. Investigations showed a normal CBC (TLC-5600) but a rise in serum creatinine to 2.3 mg/dL. Ultrasound of the lesion showed a multiloculated collection with multiple septae on ventral aspect of foot, and ultrasound of the transplant kidney revealed a thick-walled collection of 4 × 2 × 1.4 cm compressing the transplant ureter with resulting hydroureteronephrosis. Pus culture from foot lesion grew E. coli and from the periureteric collection was sterile. AFB stain, AFB, and fungal cultures and gene expert for M. tuberculosis were negative. Since the patient did not improve with antibiotics, an excision biopsy of foot lesion was done. Biopsy showed palisading granulomas with multinucleated giant cells in the dermis. Short fungal hyphal forms with constriction bands [Figure 2] and brown pigment was also seen, which was confirmed by special stains. AFB stain was negative. The patient was started on conventional amphotericin B 0.8 mg/kg, which was given for 21 days. Tacrolimus and mycophenolate were decreased. The patient responded well with resolution of remnant foot swelling and of periureteric collection along with a decrease in serum creatinine to baseline of 1.6 mg/dL.
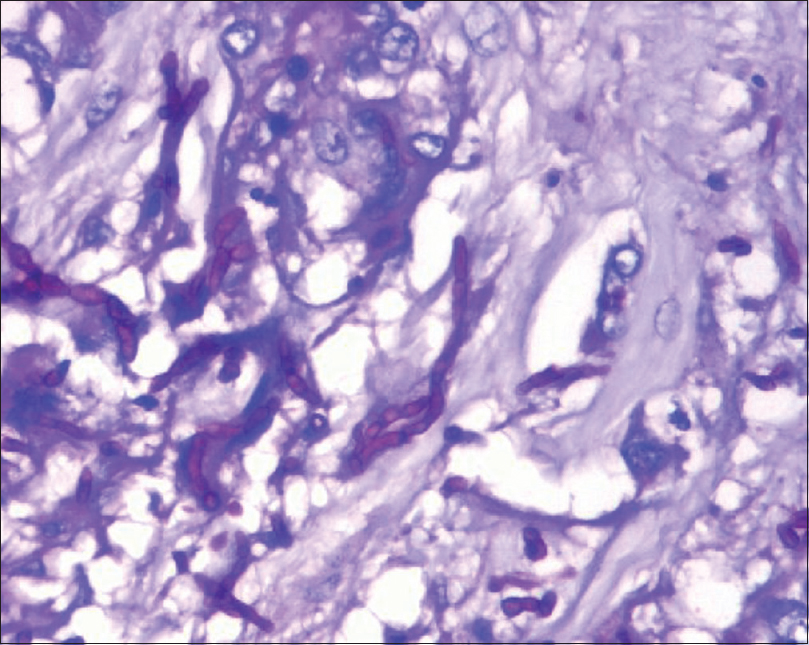
- Periodic acid–Schiff stain of phaeohyphomycosis showing septate hyphae with constrictions and bulbous ends
Case 4
A 52-year-old diabetic male presented 17 months post-kidney transplantation with left-sided facial pain in the maxillary area along with left-sided headache for 3 months. He had no history of nasal obstruction/discharge/epistaxis/fever/weight loss. On examination, deviated nasal septum to right was present and minimal mucopurulent discharge was present on the left side. Bilateral maxillary sinuses were tender to palpation. He was given oral antibiotics and planned for a functional endoscopic sinus surgery. Uncinectomy and left middle meatus antrostomy was done. Inflamed mucosa with a purulent discharge and a solid mass lesion was found in the left maxillary sinus which was sent for histopathological examination. Biopsy showed intense neutrophilic and eosinophilic infiltration of mucosa with fungal elements. Septate hyphae showing pigmentation and bulbous ends were seen.
The patient was started on conventional Amphotericin B which was given for 1 week and then shifted to oral voriconazole which was given for 4 weeks. Tacrolimus dose was decreased and monitored. The patient improved with a complete resolution of symptoms and with stable graft function.
Case 5
A 52-year-old male case presented 1 month after his kidney transplantation with loose stools and decreased urine output. He also had a wound on the left hallux with some pus discharge from it. Investigations showed a TLC of 5600 cells/mm3 (90% neutrophils) and a rise in serum creatinine from baseline of 1.7 to 2.1 mg/dL. Stool routine microscopy and culture were normal. Whole blood CMV PCR was positive with 21,000 copies/mL. Pus for fungal stain from foot lesion showed hyphal forms on KOH, and an excision biopsy of the toe lesion was done. It revealed focal areas of abscess and granuloma formation with septate pigmented fungal hyphae likely phaeohyphomycosis [Figure 3]. The patient was given ganciclovir for 21 days and Amphotericin B for 14 days. Tacrolimus level was high (18 ng/ml) and tacrolimus dose was decreased. Patient improved with therapy with resolution of diarrhea and complete healing of toe wound.
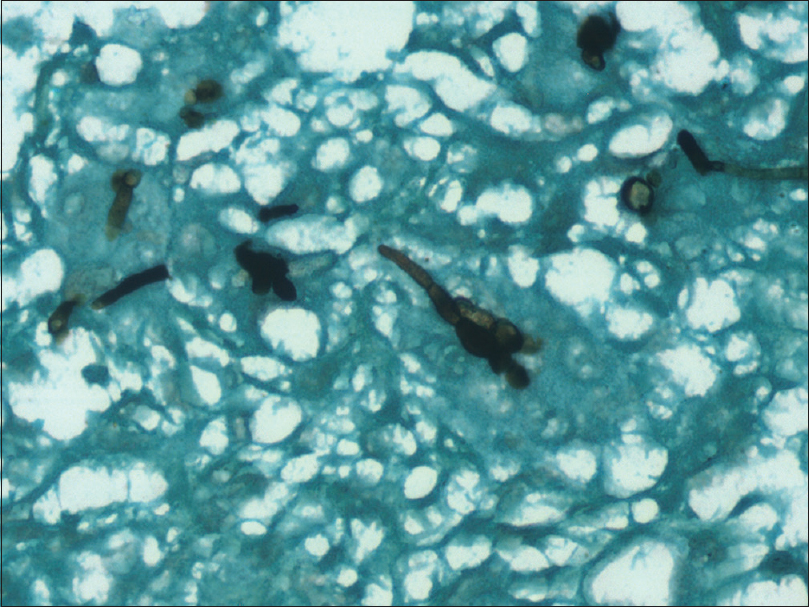
- Gomori-Methanamine stain of phaeohyphomycosis highlighting short hyphal forms and bulbous spores
Table 1 summarizes the clinical details of the cases.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age (in years) | 50 | 55 | 35 | 52 | 52 |
| Gender (M=Male) | M | M | M | M | M |
| Site of infection | Popliteal fossa, foot | Pulp space of right second distal phalanx, dorsum of foot | Sole of foot, perinephric collection | Left maxillary sinus | Left hallux |
| Number of lesions | two | two | two | one | one |
| Basic kidney disease | CIN | CGN | CGN | Diabetic nephropathy | CGN |
| Type of transplant | LRRT | DDRT | LRRT | LRRT | LRRT |
| Time from transplantation | 24 months | 3 months | 24 months | 17 months | 1 month |
| Induction agent | ATG | ATG | ATG | Basiliximab | ATG |
| Maintenance agent | Tacrolimus + MMF + steroid | Tacrolimus + MMF + steroid | Tacrolimus + MMF + steroid | Everolimus + tacrolimus+ MMF + steroid | Tacrolimus + MMF + steroid |
| Pre-existing Comorbidities | Chronic hepatitis C (treated) | none | Chronic allograft injury | Type 2 DM | none |
| Past h/o antibody-mediated rejection | |||||
| Co-infection | none | Acinetobacter species | E coli | none | CMV viremia |
| Graft dyfunction | absent | present | present | present | present |
| Treatment | Itraconazole + partial surgical excision | Voriconazole/caspofungin + Incision and drainage + excision of one lesion | Amphotericin B + complete excision | Amphotericin + voriconazole + excision | Amphotericin B + complete excision |
| Outcome | Partial regression | Death | Complete recovery | Complete recovery | Complete recovery |
CGN=Chronic glomerulonephritis, CIN=chronic interstitial nephritis, ATG=antithymocyte globulin, MMF=mycophenolate mofetil, CMV=cytomegalovirus, LRRT=live related renal transplant, and DDRT=deceased donor renal transplant
Discussion
Phaeohyphomycosis is caused by dematiaceous fungi, which are fungi with pigment/melanin in their cell wall.[2] They are rare infections constituting around 2.5% of all invasive fungal infections in solid organ transplant recipients. Although they predominantly cause cutaneous infections, they are notorious for causing deep-seated invasive disease including pulmonary, sinus, and blood stream infections in immunocompromised individuals.[3] Most of our patients presented with cutaneous manifestations with disseminated cutaneous lesions in 50%. Two had rare sites of infection – one patient had a sinusitis and one had a periureteric collection.
The time since transplantation was a median of 510 days (32–720 days). Since this fungus is widely distributed in nature and acquired by inhalation or skin trauma, it is often found >6 months post transplantation.[2] Only one of our patients had a history of skin trauma while being involved in agricultural work in rice fields.
Histopathological analysis of excised tissue led to the diagnosis in 100% of cases. It is to be noted that fungal culture may not always be positive (0% in our study) but is important for species identification. In another study from South India, culture reports did not grow fungi in 71.4% cases.[4] Hence, it is important to send histopathological analysis of specimens from all cases of suspected fungal infections. Gomori-Methanamine stain, Periodic Acid Schiff, and Fontana-Masson stains are important in delineating these pigmented fungi on pathological specimens and should be used whenever there is a suspicion of phaeohyphomycosis.[1] Characteristic fungal hyphae are septate and thin but have irregular swellings and constrictions. Ends or intercalated areas may show vesicular swelling with thick walls.[5]
Treatment of phaeohyphomycosis varies with clinical presentation, underlying condition of the patient and the species identified.[3] Early therapy with a combination of surgical intervention, antifungals, and appropriate modification of immunosuppression is of key importance to improve outcomes in patients. Patients with localized subcutaneous lesions have a dramatic response to surgical therapy alone but may be given concomitant antifungal therapy in view of the immunosuppressed state. Where lesions are disseminated or multiple, antifungals should be administered for 3–12 months. Azole antifungals (itraconazole, voriconazole, and posaconazole) have been suggested as first line agents. Amphotericin B may be used in deep-seated or resistant infections.[67]
All our patients received systemic antifungal therapy (azoles/amphotericin/caspofungin) and excision of lesions where possible. Duration of antifungal in our cases was short owing to good response to surgical excision in most cases. Reduction of immunosuppression was done in all our patients with decrease in dose of antiproliferative agent and decrease in tacrolimus doses. A complete withdrawal of immunosuppression was done in one patient who had disseminated infection and ultimately succumbed to his infection.
Patients with phaeohyphomycosis with deep-seated infections have a higher mortality rate than patients with isolated cutaneous lesions.[4] Among our cases, we lost one patient who had disseminated infection on Day 28 of disease. However, two patients with deep-seated infection (periureteric and sinus infection) recovered with antifungal therapy and excision with complete recovery.
Conclusion
Phaeohyphomycosis is an emerging menace in the realm of renal transplantation. It is important to have a high index of suspicion of fungal infection in patients with indolent skin lesions and abscesses which do not respond to antibacterials. Transplant recipients may also have unusual deep-seated infections. Appropriate microbiological and histopathological evaluation of lesions helps in establishing a diagnosis. Response to complete surgical excision is remarkable, but it should be accompanied by antifungal therapy in immunocompromised patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin Microbiol Infect. 2014;20((Suppl 3)):47-75.
- [Google Scholar]
- Phaeohyphomycosis in transplant recipients: Results from the Transplant Associated Infection Surveillance Network (TRANSNET) Med Mycol. 2015;53:440-6.
- [Google Scholar]
- Subcutaneous phaeohyphomycosis in kidney transplant recipients: A series of seven cases? Transpl Infect Dis 2017:19. doi: 10.1111/tid. 12788
- [Google Scholar]
- Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-80.
- [Google Scholar]
- ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin Microbiol Infect. 2014;20((Suppl 3)):47-75.
- [Google Scholar]
- Emerging fungal infections in solid organ transplantation. Am J Transplant. 2013;13((Suppl 4)):262-71.
- [Google Scholar]







